Osivax influenza vaccine is making waves in the biopharmaceutical industry with its innovative approach to combating influenza A viruses. The company, based in France, has recently secured a substantial $19.5 million contract from the Biomedical Advanced Research and Development Authority (BARDA) aimed at developing a broad-spectrum flu vaccine. This investment not only focuses on pandemic flu preparedness but also positions Osivax’s OVX836 vaccine as a leading contender for seasonal flu solutions. With a unique design that targets the highly conserved nucleoprotein (NP) of the influenza A virus, this candidate vaccine shows promise for eliciting a robust immune response. The financial backing from BARDA is set to enhance the clinical development and large-scale trial readiness necessary for this groundbreaking vaccine’s success.
Introducing the cutting-edge vaccine being developed by Osivax, a French biopharmaceutical firm, which aims to transform the way we fight influenza A. Known as the OVX836 vaccine, this promising candidate is part of a larger effort to improve pandemic flu preparedness through a broad-spectrum approach. With substantial funding from the Biomedical Advanced Research and Development Authority (BARDA), Osivax is paving the way for innovative solutions to seasonal and pandemic influenza challenges. This strategic development focuses on the nucleoprotein component of the virus, potentially enhancing immune responses with fewer mutations. As the global demand for effective flu vaccines increases, Osivax positions itself at the forefront of influenza prevention efforts.
Understanding the Osivax Influenza Vaccine: OVX836
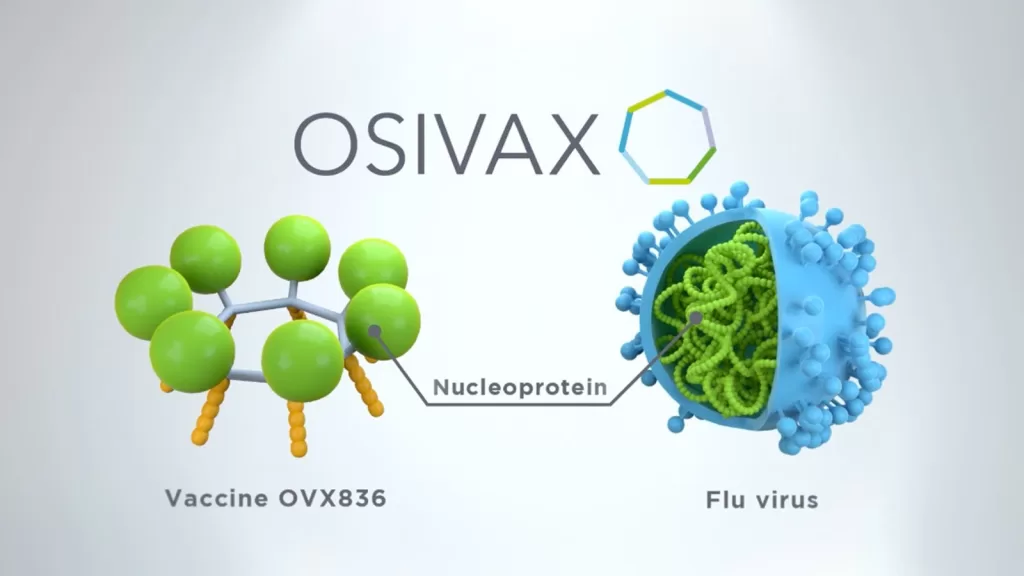
The Osivax influenza vaccine, known as OVX836, represents a significant advancement in the field of virology and immunology. It is specifically engineered to target the nucleoprotein of the influenza A virus, a strategy that not only aims to deliver a robust immune response but also enhances the vaccine’s resilience against mutations. As researchers work toward developing a broad-spectrum flu vaccine, OVX836 has become a focal point for pandemic flu preparedness due to its unique design and mechanism of action, which significantly amplifies its potential efficacy against various strains of the virus.
Recent studies involving OVX836 have shown promising results in clinical trials, with a diverse group of 1,400 participants demonstrating satisfactory safety profiles and strong immunogenicity. This indicates that the vaccine is not only safe for human use but also elicits adequate immune responses necessary to combat infections that have historically challenged public health systems. The support from BARDA, with a contract valued at $19.5 million, underscores the importance of OVX836 in addressing both seasonal flu and potential pandemic threats.
The Role of BARDA in Flu Vaccine Development
The Biomedical Advanced Research and Development Authority (BARDA) plays a crucial role in the landscape of biopharmaceuticals, particularly in the context of influenza vaccine development. With mandates that encompass pandemic preparedness, BARDA provides vital funding and resources that help accelerate the research and clinical development of candidate vaccines like OVX836. This partnership is essential for companies like Osivax, enabling them to navigate the complex processes of vaccine development, from early-stage trials to readiness for large-scale manufacturing.
In 2023, BARDA’s $19.5 million contract for OVX836 is not just a financial investment; it is a strategic commitment to enhancing national health security. By backing clinical development and preparing for large efficacy studies, BARDA ensures that innovative solutions to combat influenza are streamlined and that biopharmaceutical entities can effectively respond to outbreaks. This proactive approach is vital for maintaining preparedness against future pandemics, particularly as the landscape of viral threats continues to evolve.
Developing a Broad-Spectrum Flu Vaccine for the Future
The ongoing pursuit of a broad-spectrum flu vaccine is critical as seasonal influenza and potential pandemic strains pose continual threats to global health. Current vaccine paradigms often struggle to keep pace with rapidly mutating viruses, highlighting the need for innovative solutions like OVX836. This candidate vaccine’s approach to target the highly conserved nucleoprotein of the influenza A virus promises to provide a more reliable immune response, potentially mitigating the impact of diverse influenza strains.
Furthermore, developing a broad-spectrum vaccine aligns with international public health goals to enhance pandemic flu preparedness, particularly in regions that may be vulnerable to severe outbreaks. The unique properties of OVX836 serve as a foundational element in this larger strategy, facilitating advancements in vaccine technology. By focusing on creating a vaccine that can address uncertainties associated with influenza, Osivax is contributing significantly to global readiness for future influenza seasons.
The Innovation Behind the OVX836 Vaccine Technology
Osivax’s approach with the OVX836 vaccine is anchored in innovative biopharmaceutical technology that utilizes a recombinant nucleoprotein that self-assembles into nanoparticles. This cutting-edge method not only increases the stability of the vaccine but also enhances its ability to stimulate both T-cell and B-cell immune responses. Such a comprehensive immune activation is crucial in ensuring that the body can adequately respond to actual influenza infections, a feature that is often missing in traditional vaccine designs.
Incorporating technology that enhances immune responses marks a significant shift away from conventional vaccines that solely rely on surface antigens. As the need for broadly effective vaccines becomes more pressing in light of emerging viral variants, the OVX836 model stands out. Its advanced technological framework positions it favorably as a candidate not just for seasonal influenza but also for any unforeseen pandemics, making the research and development efforts led by Osivax highly relevant.
Influenza A Candidate Vaccines and Their Importance
Influenza A candidate vaccines, such as OVX836, are pivotal in controlling outbreaks at both the seasonal and pandemic levels. The unique properties of these candidate vaccines allow for the design of immunizations that can respond more flexibly to the antigenic changes that characterize influenza A viruses. This adaptability becomes particularly significant when addressing novel strains that may emerge, necessitating a more comprehensive defense strategy.
With the rapid evolution of viruses, harnessing the potential of candidate vaccines helps in not only preparing for known threats but also in anticipating future challenges. Candidate vaccines like OVX836 represent a robust line of defense, showcasing the importance of continuous investment in innovative vaccine solutions. By embracing such advancements, health authorities and biopharmaceutical companies can build a resilient framework against influenza A virus, ensuring effective responses in the face of pandemics.
Clinical Trials: The Pathway to Market for OVX836
The clinical trial process is a critical pathway that vaccines like OVX836 must navigate before reaching the market. The initial phases of clinical studies provide invaluable data concerning the vaccine’s safety and efficacy. The participation of 1,400 individuals in these trials indicates a robust engagement with the community while also aiding researchers in gathering comprehensive immunogenicity data. This step is essential for ensuring that when the vaccine becomes available, it meets the high standards necessary for public distribution.
Successful completion of these trials not only builds confidence among users and healthcare professionals but also paves the way for the subsequent phases that will determine large-scale production capabilities. With BARDA’s backing, Osivax is positioned to utilize the insights gained from clinical trials to advance into larger efficacy studies. Such momentum is necessary for optimizing manufacturing processes and thereby ensuring that the distribution of OVX836 can meet future demands.
Pandemic Flu Preparedness: Lessons from the Past
Pandemic flu preparedness has emerged as a critical component of global health discussions, especially in light of recent viral outbreaks. The lessons learned from past pandemics emphasize the urgency in developing versatile vaccines like OVX836 that can provide comprehensive coverage against various strains of influenza A. The proactive investment in such vaccines reflects a commitment to safeguarding public health through advanced scientific solutions.
The strategic focus of traditional flu vaccines on specific strains has often left gaps that could be exploited by emerging variants. However, by targeting conserved antigens such as the nucleoprotein, the OVX836 vaccine aims to close those gaps, making it a cornerstone in future preparedness strategies. This approach not only enhances individual health responses but also contributes to global strategies aimed at controlling viral spread during pandemic scenarios.
Biopharmaceutical Contracts and Their Impact on Vaccine Development
Biopharmaceutical contracts, such as the one awarded to Osivax by BARDA, signify a crucial investment in public health. These contracts provide necessary funding and direction for the research and development of innovative vaccines that can mitigate the effects of influenza. The $19.5 million contract specifically allocated for OVX836 underscores the importance of such partnerships in advancing vaccine technology and ensuring that preparedness for pandemic flu is prioritized.
The impact of these contracts goes beyond financial support; they also facilitate knowledge sharing, access to expertise, and streamlined development pathways. By collaborating with governmental entities like BARDA, biopharmaceutical companies can leverage resources and stand to gain insights that enhance their operational efficiencies and lead to faster time-to-market for life-saving vaccines. This collaborative model encourages innovation and expedites the response to public health threats.
The Future of Influenza Vaccination: Trends and Innovations
Looking ahead, the future of influenza vaccination is likely to be shaped by trends that prioritize advanced technologies and comprehensive strategies. Innovations like the OVX836 vaccine exemplify how biopharmaceutical companies are increasingly focusing on developing vaccines that can effectively respond to a rapidly changing viral landscape. As scientific research continues to evolve, newer, more effective vaccination strategies will likely emerge.
Moreover, the adaptation to global health needs will necessitate flexibility in vaccine development approaches. This includes building upon the model established by OVX836, which emphasizes broad-spectrum responses and minimized viral escape. As the field progresses, integrating lessons learned from current and past efforts will be essential for ensuring that flu vaccines not only meet immediate needs but also anticipate future public health challenges.
Frequently Asked Questions
What is the Osivax influenza vaccine and how does it work?
The Osivax influenza vaccine, also known as the OVX836 vaccine, is a broad-spectrum influenza A candidate vaccine designed to trigger a robust immune response against the influenza virus. It specifically targets the nucleoprotein (NP) of the virus, which is a highly conserved internal antigen. This design helps ensure the vaccine is less likely to mutate, potentially offering better protection against various strains of influenza.
How does the OVX836 vaccine contribute to pandemic flu preparedness?
The OVX836 vaccine plays a crucial role in pandemic flu preparedness as it is designed to provide broad protection against multiple strains of the influenza A virus. With its focus on a conserved target, it aims to generate a more universal immune response, which is essential for mitigating the impact of future influenza pandemics. The recent contract with BARDA supports the vaccine’s development for both pandemic and seasonal flu preparedness.
What funding has Osivax received for the development of the OVX836 vaccine?
Osivax has received a $19.5 million contract from the Biomedical Advanced Research and Development Authority (BARDA) to advance the development of its OVX836 vaccine. This funding includes $11.5 million for clinical development and $8 million for preparations for large-scale efficacy studies, ensuring the vaccine’s readiness for extensive trials.
What is the significance of the BARDA contract for the Osivax influenza vaccine?
The BARDA contract is significant for the Osivax influenza vaccine as it provides vital financial support for ongoing clinical trials and large-scale manufacturing preparations. This partnership underscores the importance of the OVX836 vaccine in addressing both seasonal and pandemic influenza threats, enhancing public health safety.
What are the early clinical trial results for the OVX836 vaccine?
Early clinical trials of the OVX836 vaccine have involved approximately 1,400 participants and have shown promising results regarding the vaccine’s safety and immunogenicity. These encouraging data suggest that the vaccine could effectively stimulate immune responses, supporting its potential use against influenza A infections.
How does the design of the OVX836 vaccine enhance its effectiveness?
The design of the OVX836 vaccine enhances its effectiveness by utilizing a recombinant nucleoprotein (NP) that self-assembles into nanoparticle structures. This innovative approach boosts both T- and B-cell immune responses, potentially offering a more comprehensive defense against the influenza A virus, making it a standout candidate in the development of broad-spectrum flu vaccines.
| Key Points | |
|---|---|
| Company | Osivax, a biopharmaceutical firm based in France. |
| Contract Value | $19.5 million from BARDA for influenza vaccine development. |
| Vaccine Name | OVX836, designed to combat influenza A. |
| Funding Allocation | $11.5 million for clinical development; $8 million for efficacy study preparations. |
| Vaccine Target | Targets nucleoprotein (NP) of the influenza A virus, aiming for a universal immune response. |
| Clinical Trials | Promising safety and immunogenicity data from early trials involving 1,400 participants. |
Summary
The Osivax influenza vaccine, OVX836, represents a significant advancement in flu vaccine technology. With its recent $19.5 million government contract, Osivax is set to enhance pandemic and seasonal flu preparedness. By focusing on the highly conserved nucleoprotein of the influenza A virus, OVX836 aims to create a broader immune response, potentially increasing vaccine effectiveness. Ongoing trials have already shown promising results, showcasing the vaccine’s safety and ability to invoke a strong immune response. As developments progress, the Osivax influenza vaccine could become an essential tool in global health management against flu outbreaks.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.