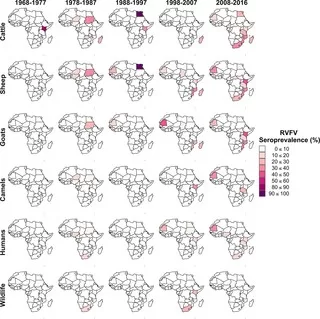
Understanding the seroprevalence of Rift Valley Fever Virus (RVFV) provides vital insights into zoonotic viral infections and their impact on public health, particularly in regions like Benin. Recent research highlights that, despite a lack of positive PCR results among 650 febrile patients screened during 2022-2023, the presence of virus-specific IgG antibodies indicates that RVFV is circulating in the population. This finding is crucial, as RVFV and Crimean-Congo hemorrhagic fever virus (CCHFV) can cause severe disease in humans and livestock. Moreover, the study emphasizes the challenges of serologic testing in malaria-endemic areas, where false-positive results from malaria-associated antibodies can skew data. Therefore, ongoing surveillance and improved serologic validation are essential for effective epidemiology and management of RVFV and related zoonotic viral infections in West Africa.
The detection of antibodies associated with the Rift Valley fever virus (RVFV) illustrates a significant public health concern regarding viral infections transmitted from animals to humans, commonly referred to as zoonoses. In recent investigations, particularly in the context of Benin, the epidemiological landscape shows an underappreciated prevalence of RVFV, alongside the recognized threats of Crimean-Congo hemorrhagic fever (CCHF). As the region grapples with changing agricultural practices and increased livestock farming, the surveillance for such viruses becomes increasingly critical. Furthermore, the intersection of RVFV research with serologic testing for malaria highlights a broader need for precision in diagnostic methodologies that can differentiate viral infections accurately. Addressing these complexities is paramount to inform effective health policies and vaccination strategies in combating RVFV and CCHF in African communities.
Understanding Rift Valley Fever Virus Seroprevalence in Benin
Rift Valley fever virus (RVFV) is a critical concern in Benin, given its potential to cause zoonotic outbreaks that affect both livestock and human populations. The seroprevalence study conducted between 2022 and 2023 highlighted that while RVFV is endemic to the region, the actual infection rates among febrile patients remain low, with only 1.5% testing positive for RVFV-specific IgG antibodies. Such low seroprevalence rates may indicate limited recent exposure or a significant underreporting of cases due to asymptomatic infections.
The implications of these findings are dire, particularly as the livestock sector in Benin is undergoing significant changes. Increasing herd sizes and shifting farming practices can facilitate RVFV transmission by altering the interactions between vectors and hosts. Continuous monitoring and serologic testing for RVFV in both humans and livestock are imperative to understanding the epidemiological trends and implementing effective preventative measures.
Frequently Asked Questions
What is the seroprevalence of Rift Valley Fever Virus in Benin as reported recently?
The recent study reported a seroprevalence of 1.5% for Rift Valley Fever Virus (RVFV) among febrile patients in Benin, indicating some level of circulation of the virus despite no positive results from PCR testing.
How does Crimean-Congo Hemorrhagic Fever virus relate to Rift Valley Fever virus seroprevalence in Benin?
The study indicated that 6.1% of samples tested positive for Crimean-Congo Hemorrhagic Fever Virus (CCHFV) using indirect ELISA. This highlights the need for comprehensive monitoring of both RVFV and CCHFV in the region.
What are the implications of serologic testing for Rift Valley Fever Virus in malaria-endemic areas like Benin?
In malaria-endemic areas such as Benin, serologic testing for RVFV can yield false positives due to cross-reactivity with malaria antibodies. This emphasizes the importance of validating serological tests for accurate diagnosis in these regions.
What risks do livestock farming changes pose for Rift Valley Fever Virus circulation in Benin?
Changes in traditional livestock farming, like increased herd sizes and sedentarization, may enhance the circulation of Rift Valley Fever Virus (RVFV) and Crimean-Congo Hemorrhagic Fever Virus (CCHFV), leading to higher risks of zoonotic transmission.
What methods are used for serological detection of Rift Valley Fever Virus and Crimean-Congo Hemorrhagic Fever in Benin?
The study employed ELISA kits for RVFV and CCHFV serological detection, complemented by indirect IgG immunofluorescence assays (IFAs) for confirmation, underlining the necessity for accurate diagnostic methods in regions like Benin.
Why is monitoring Rift Valley Fever Virus seroprevalence important in Benin?
Monitoring seroprevalence of Rift Valley Fever Virus (RVFV) and Crimean-Congo Hemorrhagic Fever Virus (CCHFV) is crucial to understand viral circulation in both humans and livestock, which can inform vaccination strategies and public health responses across sub-Saharan Africa.
What factors contributed to the low seroprevalence rates of Rift Valley Fever Virus in Benin?
Factors contributing to low RVFV seroprevalence rates in Benin may include the absence of detectable acute infection cases through PCR methods and the challenges of cross-reactivity due to endemic malaria, necessitating careful interpretation of serological data.
| Key Points |
|---|
| Rift Valley Fever Virus (RVFV) and Crimean-Congo Hemorrhagic Fever Virus (CCHFV) are endemic to Africa and the Arabian Peninsula, causing serious health threats. |
| A study conducted in Benin from 2022 to 2023 screened 650 febrile patients for RVFV and CCHFV. |
| No positive results were found via PCR; however, 1.1% had RVFV IgG and 0.3% had CCHFV IgG. |
| False-positive serological results may arise from malaria-related antibodies and highlight the need for careful validation of tests in malaria-endemic regions. |
| High rates of unspecific reactivity indicate challenges in serologic testing and the importance of enhancing sensitivity and specificity in endemic areas. |
| The circulation of RVFV and CCHFV, albeit at low rates, emphasizes the necessity of continual monitoring and improved serology testing methods. |
| Robust epidemiological data is critical for effective vaccination strategies for both livestock and humans in sub-Saharan Africa. |
Summary
Rift Valley Fever Virus Seroprevalence is a crucial topic illustrated by a study conducted in Benin, where the low detection rates of antibodies against RVFV and CCHFV were documented. The findings underscore the existence of these viruses in the region, although at low seroprevalence rates. The study highlights significant methodological challenges posed by false positives due to malaria, necessitating rigorous validation of serologic tests. These outcomes reinforce the critical need for ongoing surveillance and improved testing methods to better understand and manage the epidemiology of these lethal viruses in West Africa.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.