The emergence of a new oral gonorrhea antibiotic called zoliflodacin has captured the attention of health professionals and patients alike. This innovative treatment, pending FDA approval, promises to be a crucial addition in the fight against gonorrhea, which is responsible for approximately 82 million new infections a year globally. Due to rising antibiotic resistance among current treatment options, including ceftriaxone, the introduction of zoliflodacin comes at a critical time. This first-in-class antibiotic offers hope for effective gonorrhea treatment, particularly against drug-resistant strains that pose significant health risks, such as infertility and ectopic pregnancies. With the potential to reshape gonorrhea treatment options, the FDA’s review of zoliflodacin holds great promise for addressing the challenges of sexually transmitted infections in the modern era.
In light of rising concerns about sexually transmitted infections, an exciting new contender has emerged in the form of zoliflodacin, a revolutionary oral treatment for gonorrhea. As we continue to face challenges with antibiotic resistance, this investigational drug represents a leap forward in gonorrhea management. The escalating rates of gonorrhea infections, along with limited effective treatments, underscore the urgent need for innovative solutions. This new oral antibiotic could offer an essential lifeline for individuals battling uncomplicated gonorrhea, particularly those affected by resistant strains. With its distinct mechanism and potential for FDA approval, zoliflodacin may soon become a vital tool in combating the growing epidemic of sexually transmitted diseases.
Understanding Oral Gonorrhea Antibiotics
Oral gonorrhea antibiotics are at the forefront of the battle against one of the most prevalent sexually transmitted infections (STIs) worldwide. As antibiotic resistance continues to rise, particularly against traditional treatments like ceftriaxone, the need for effective oral alternatives is critical. The recent FDA review of zoliflodacin, a novel oral antibiotic, is a significant step toward addressing this urgent public health concern. As the second most reported STI globally, gonorrhea affects millions annually, highlighting the urgency of innovative treatments.
The FDA’s acceptance of the new drug application (NDA) for zoliflodacin comes at a time when healthcare providers are in dire need of effective strategies to combat the increasing prevalence of gonorrhea. The oral formulation of zoliflodacin not only offers convenience but also targets strains of *Neisseria gonorrhoeae* that have become resistant to conventional therapies. This persistence of antibiotic-resistant infections emphasizes the importance of developing new therapeutic options to adapt to the evolving landscape of STIs.
The Role of Zoliflodacin in Gonorrhea Treatment
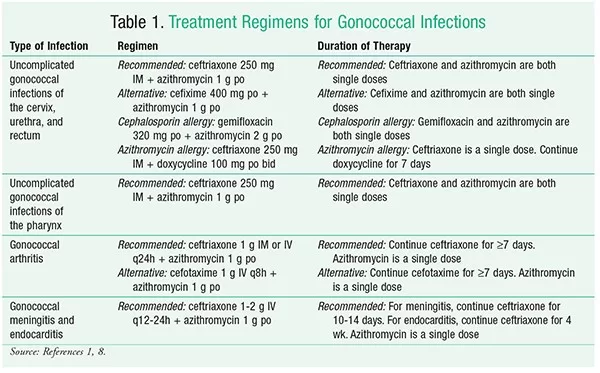
Zoliflodacin stands out as a first-in-class antibiotic with a unique mechanism of action that differentiates it from existing treatments. Clinical trials have demonstrated that a single dose of zoliflodacin can effectively cure uncomplicated gonorrhea, offering hope for a streamlined treatment regimen that mitigates the need for multi-drug therapies. By providing an alternative to ceftriaxone and azithromycin, zoliflodacin could help reduce the selection pressure that often leads to further antibiotic resistance among gonococcal strains.
Moreover, zoliflodacin’s efficacy extends to multidrug-resistant strains, making it a potentially invaluable tool in the arsenal against gonorrhea. The collaboration between Innoviva and the Global Antibiotic Research & Development Partnership ensures that once FDA approval is granted, zoliflodacin can reach diverse populations, particularly in low- and middle-income countries where access to effective treatments is limited. This strategic partnership underscores the importance of global health initiatives in addressing public health challenges posed by STIs.
Challenges of Antibiotic Resistance in Gonorrhea
The growing issue of antibiotic resistance remains a major hurdle in the treatment of gonorrhea. As the bacteria adapt and evolve, treatment options become increasingly limited, placing pressure on healthcare systems and exacerbating public health risks. The advancing resistance against standard therapies like ceftriaxone, which was once the backbone of gonorrhea treatment, necessitates the urgent development of new antimicrobial agents like zoliflodacin to address treatment failures and control the spread of this infection.
Public awareness and education are crucial components in combating the rise of antibiotic-resistant gonorrhea. By promoting safe sexual practices and encouraging regular testing, healthcare providers can help mitigate the spread of STIs and the emergence of resistant strains. Investment in research and development, alongside effective public health strategies, will be essential in overcoming the challenges posed by antibiotic resistance and ensuring effective treatment options remain available for future generations.
Innovations in Gonorrhea Treatment Landscape
The introduction of innovative antibiotics such as zoliflodacin heralds a new era in the treatment landscape for gonorrhea. As researchers continue to explore novel mechanisms of action, the potential for developing effective therapies that can outpace bacterial resistance becomes tangible. Alongside zoliflodacin, other candidates like gepotidacin, which have shown promising efficacy against gonorrhea, illustrate a trend toward diversification in treatment options for STIs.
Such innovations are vital in responding to the global health crisis posed by sexually transmitted infections. The ongoing research and development efforts fueled by public-private partnerships aim to not only enhance treatment but also ensure these advancements are widely accessible. As the FDA evaluates the safety and efficacy of these new oral antibiotics, the hope is that we can soon provide patients with more effective and user-friendly options that meet the demands of modern healthcare.
Future Directions for Gonorrhea Antibiotic Research
As the urgency for effective gonorrhea treatment escalates, future research directions will likely focus on addressing the complexities of antibiotic resistance. Investigations into the genetic mechanisms behind *Neisseria gonorrhoeae* resistance, as well as the development of new antibiotics like zoliflodacin, will be crucial. In addition, the exploration of combination therapies may present opportunities to enhance treatment outcomes and reduce resistance rates.
Moreover, funding initiatives aimed at antibiotic discovery and development will be essential in ensuring that new treatments become available to combat both established and emerging strains of resistance. Collaboration among pharmaceutical companies, researchers, and public health organizations will play a pivotal role in facilitating these advancements and safeguarding public health against the backdrop of rising antibiotic resistance.
The Impact of STI Public Awareness Campaigns
Public awareness campaigns about sexually transmitted infections (STIs) are critical in preventing the spread of illnesses like gonorrhea. By educating communities on symptoms, prevention strategies, and the importance of regular screenings, we can significantly reduce the incidence of these infections. Increased awareness also helps destigmatize STIs, encouraging individuals to seek treatment and discuss their sexual health openly.
Moreover, these campaigns can highlight the dangers of antibiotic resistance, emphasizing the importance of using antibiotics appropriately. Educating the public about completing prescribed treatments and the risks associated with self-medication or untreated infections can foster a culture of responsible health behaviors, ultimately aiding in the fight against gonorrhea and other STIs.
Emerging Trends in Gonorrhea Treatment Protocols
The emergence of new treatment protocols incorporating innovative antibiotics like zoliflodacin reflects a dynamic response to the evolving landscape of gonorrhea management. As antibiotic resistance becomes more prevalent, healthcare providers must adapt their treatment protocols to incorporate new therapies that demonstrate efficacy against resistant strains. These emerging protocols may also consider factors such as patient compliance and the pharmacokinetics of new medications to optimize treatment outcomes.
Integration of zoliflodacin and other novel therapies into standard treatment guidelines could revolutionize the approach to treating uncomplicated gonorrhea. By evaluating real-world data on patient responses and resistance patterns, health authorities can refine these protocols to ensure they remain effective in the face of an ever-changing bacterial landscape.
Health Policy Implications of New Gonorrhea Antibiotics
The FDA’s review of zoliflodacin and its potential approval carries significant health policy implications as countries grapple with increased STI rates and antibiotic resistance. Policymakers must prioritize access to new medications to ensure that they reach populations at risk of untreated gonorrhea, particularly among vulnerable demographics in lower-income regions. Additionally, public funding initiatives may be required to facilitate the distribution of these essential treatments.
Effective health policy must also encompass ongoing surveillance of gonorrhea treatment outcomes to assess the long-term efficacy of new antibiotics like zoliflodacin. By integrating data collection and analysis into health systems, policymakers can make informed decisions to enhance treatment protocols, allocate resources effectively, and ultimately contribute to the global fight against STIs and antimicrobial resistance.
The Importance of Clinical Trials in Antibiotic Development
Clinical trials are the cornerstone of antibiotic development and play a vital role in establishing the safety and efficacy of new treatments for conditions like gonorrhea. The successful phase 3 trial of zoliflodacin serves as a testament to rigorous scientific inquiry, demonstrating the potential of this new oral antibiotic to provide effective treatment for uncomplicated infections. Such trials not only validate new therapies but also enhance our understanding of dosing and patient responses.
Engaging diverse populations in clinical trials is essential to ensure that newly approved antibiotics are effective across different demographics. Lessons learned from these trials can provide insights into the diverse experiences of patients and help tailor treatment protocols to meet varied needs. Furthermore, expanding participation in clinical trials can accelerate the development timeline of new compounds, bringing much-needed therapies to market sooner.
Frequently Asked Questions
What is Zoliflodacin, and how does it relate to oral gonorrhea treatment?
Zoliflodacin is an investigational oral antibiotic specifically designed for the treatment of uncomplicated gonorrhea. As the first new antibiotic for gonorrhea in decades, it offers hope against the increasing antibiotic resistance faced with current treatments. If approved by the FDA, it could provide an effective alternative for patients suffering from this common sexually transmitted infection.
Why is the FDA review important for the new oral gonorrhea antibiotic, Zoliflodacin?
The FDA review of Zoliflodacin is crucial because it may lead to the approval of a novel oral antibiotic that targets gonorrhea, addressing the urgent need for new treatments amidst rising antibiotic resistance. This could significantly enhance the treatment options available for patients and healthcare providers managing uncomplicated gonorrhea infections.
How does Zoliflodacin work against antibiotic-resistant gonorrhea strains?
Zoliflodacin has a unique mechanism of action that targets the *Neisseria gonorrhoeae* bacteria differently than existing antibiotics. It has shown activity against multidrug-resistant strains without cross-resistance to other treatments, making it a promising solution in the fight against antibiotic-resistant gonorrhea.
What are the potential benefits of Zoliflodacin for treating sexually transmitted infections?
If approved, Zoliflodacin could provide a powerful new treatment option for sexually transmitted infections, particularly uncomplicated gonorrhea. It may help reduce the prevalence of antibiotic-resistant infections and improve health outcomes, particularly for populations with limited access to effective treatments.
How does Zoliflodacin compare to current gonorrhea treatment regimens?
In clinical trials, Zoliflodacin demonstrated noninferiority to the standard treatment regimen of ceftriaxone plus azithromycin for achieving microbiologic cure in uncomplicated gonorrhea. It showed a favorable safety profile with no serious adverse events reported, positioning it as a viable alternative treatment.
| Key Point | Details |
|---|---|
| FDA Review | The FDA has accepted the new drug application (NDA) for zoliflodacin, a potential oral antibiotic for uncomplicated gonorrhea. |
| Importance of Zoliflodacin | If approved, it would be the first new antibiotic for gonorrhea in decades, addressing the rise of drug-resistant strains. |
| Resistance Issues | Gonorrhea is becoming increasingly resistant to ceftriaxone, raising concerns about drug-resistant and untreatable infections. |
| Clinical Trials | A phase 3 clinical trial demonstrated that zoliflodacin was noninferior in achieving microbiologic cure compared to ceftriaxone plus azithromycin. |
| Global Collaboration | Zoliflodacin is developed with the Global Antibiotic Research & Development Partnership (GARDP), emphasizing the importance of public-private partnerships. |
Summary
Oral gonorrhea antibiotics are witnessing significant advancements as the FDA considers zoliflodacin, a potentially groundbreaking treatment for uncomplicated gonorrhea. With gonorrhea infections causing over 82 million new cases each year and increasing resistance to existing treatments, the approval of zoliflodacin would mark a crucial development in combatting this widespread sexually transmitted infection. Its unique mechanism of action and the promising results from clinical trials highlight its importance. If successful, zoliflodacin could become a vital tool in the fight against multidrug-resistant gonorrhea, providing new hope to millions affected globally.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.