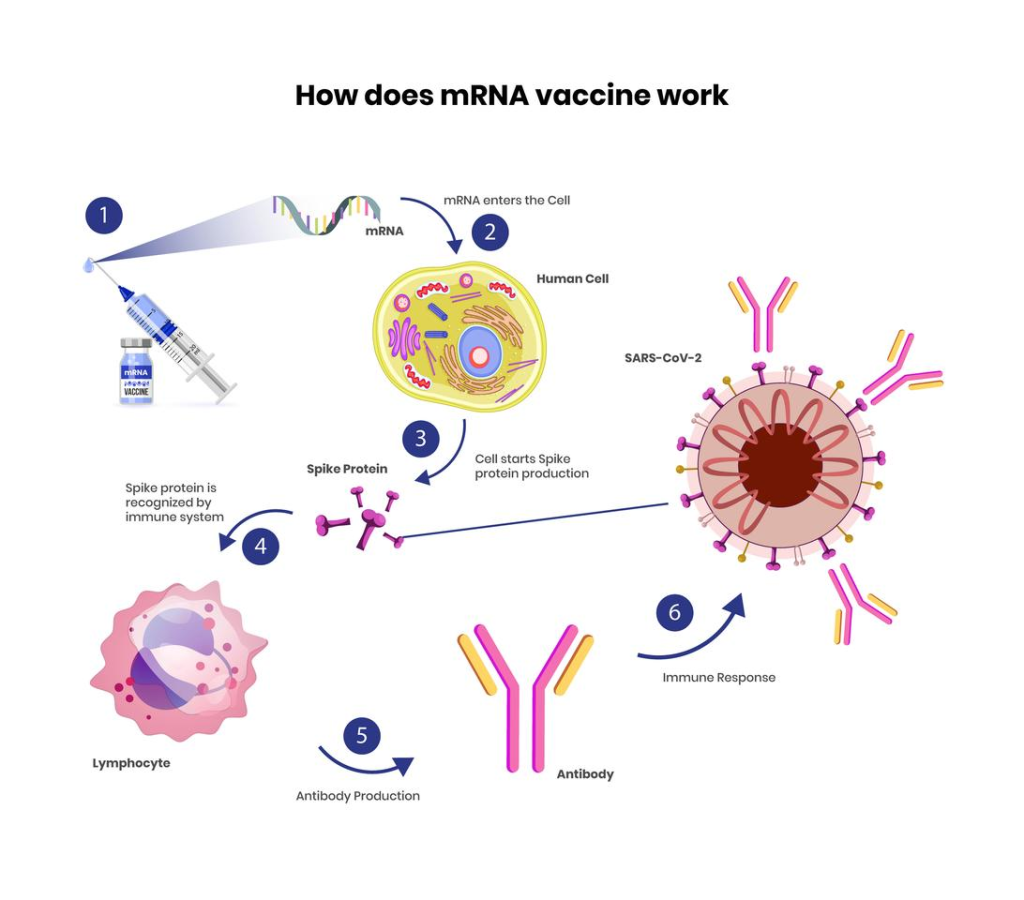
mRNA vaccines are at the forefront of modern immunization technology, particularly highlighted by the recent $590 million funding from the U.S. Department of Health and Human Services (HHS) to Moderna. This investment aims to accelerate the development of mRNA vaccines specifically designed to combat pandemic flu threats, such as H5N1 and H7N9 avian influenza. With the ongoing emphasis on pandemic preparedness, the deployment of mRNA technology could revolutionize how we respond to emerging infectious diseases. The support from HHS also reflects a broader strategy to leverage mRNA platforms for various vaccine candidates, including potential Moderna flu vaccines. As we navigate the challenges of future health crises, understanding the role of mRNA vaccines becomes increasingly essential for public health safety and efficacy.
The innovative realm of messenger RNA (mRNA) vaccination is transforming the landscape of disease prevention, especially in relation to influenza viruses. With significant backing from federal initiatives, such as the recent HHS funding aimed at enhancing pandemic response capabilities, companies like Moderna are leading the charge. This cutting-edge mRNA technology not only targets existing viral threats but also adapts to new, emergent strains, ensuring a robust defense against future pandemics. By exploring novel vaccine candidates, including those for H5N1 and H7N9, researchers are poised to provide effective solutions for global health challenges. As we delve deeper into this topic, the implications of mRNA vaccines on public health and pandemic readiness come into sharp focus.
The Importance of mRNA Vaccines in Pandemic Preparedness
mRNA vaccines have emerged as a crucial tool in the fight against infectious diseases, particularly in the context of pandemic preparedness. The recent $590 million funding awarded by the HHS to Moderna underscores the significance of developing mRNA vaccine platforms that can swiftly respond to potential flu pandemics. Unlike traditional vaccines, mRNA vaccines can be rapidly designed and produced, enabling a faster response to emerging threats such as avian influenza viruses. This capability is particularly vital as the world faces the continuous threat of new pandemics, necessitating a proactive approach to vaccine development.
Moreover, mRNA technology not only allows for quicker vaccine development but also offers the potential for greater adaptability. As viruses mutate, mRNA vaccines can be modified more easily compared to conventional methods. This adaptability is essential for addressing various strains of flu viruses, including those with pandemic potential like H5N1 and H7N9. By investing in mRNA vaccine technology, the HHS is positioning the United States to enhance its public health response and safeguard against future health crises.
Moderna’s Commitment to Developing Flu Vaccines
Moderna’s ongoing efforts to develop mRNA vaccines highlight the company’s dedication to addressing future pandemic threats. With the recent funding from HHS, Moderna is set to accelerate its research on vaccines targeting specific strains of avian flu, such as H5N1 and H7N9. The company’s approach involves not just creating effective vaccines but also conducting comprehensive clinical trials to ensure safety and efficacy. The planned phase 3 clinical trial for the H7N9 vaccine is a significant step forward in understanding how mRNA technology can be applied to combat serious viral threats.
In addition to the specific vaccines mentioned, Moderna’s strategy includes the development of up to four novel pandemic flu vaccines. This proactive approach is crucial in preparing for unforeseen outbreaks and ensuring that the healthcare system is equipped to handle sudden surges of flu cases. The collaboration with HHS and the substantial funding provided will not only bolster Moderna’s research capabilities but also contribute to a more robust framework for pandemic preparedness across the nation.
HHS Funding: A Strategic Investment in Vaccine Development
The recent $590 million investment from the HHS to Moderna represents a strategic commitment to enhancing the nation’s vaccine development capabilities. This funding, part of the Rapid Response Partnership Vehicle (RRPV) Consortium, emphasizes the federal government’s recognition of the need for rapid and effective responses to pandemic threats. By backing Moderna’s mRNA vaccine research, HHS is investing in a technology that can potentially revolutionize how we prepare for and respond to infectious diseases.
Furthermore, the funding from HHS not only facilitates immediate vaccine development but also contributes to long-term pandemic preparedness. As outlined in the HHS statement, this initiative aims to expand the applications of mRNA technology beyond just flu vaccines, potentially paving the way for future innovations in vaccine research. With the support of HHS, Moderna can enhance its clinical data and improve its response capabilities, ultimately leading to better health outcomes for the American population during future health emergencies.
Advancements in mRNA Technology and Its Applications
mRNA technology has witnessed significant advancements over the past few years, especially with its successful application in COVID-19 vaccines. These breakthroughs have laid a strong foundation for exploring its use against other infectious diseases, such as flu viruses. The recent commitment from HHS to support Moderna’s development of mRNA vaccines signifies a growing recognition of the technology’s potential to address various health threats efficiently and effectively.
As Moderna works on developing vaccines for H5N1 and H7N9 influenza, it is also exploring the broader implications of mRNA technology. This includes the potential to create vaccines targeting multiple strains simultaneously, which could be pivotal in managing future pandemics. The flexibility of mRNA vaccines allows for rapid updates based on circulating viral strains, making it a promising avenue for public health responses in an ever-changing viral landscape.
Clinical Trials: Ensuring Safety and Efficacy of mRNA Vaccines
The success of any vaccine hinges on rigorous clinical trials that determine its safety and efficacy. Moderna’s planned phase 1/2 study for its investigational pandemic flu vaccine, mRNA-1018, represents a critical step in this process. By enrolling healthy adults aged 18 and older, the company aims to gather comprehensive safety and immunogenicity data, which are essential for advancing to phase 3 trials. This meticulous approach not only helps build public trust in the vaccine but also ensures that the product meets the highest safety standards before it reaches the market.
In addition to the phase 1/2 study for mRNA-1018, Moderna’s strategy includes conducting multiple clinical trials for different pandemic flu vaccine candidates. Each trial plays a vital role in assessing the performance of these vaccines against various strains, particularly those with pandemic potential. Through this extensive research, Moderna aims to contribute valuable insights into mRNA vaccine technology, ultimately enhancing the overall landscape of vaccine development and preparedness.
The Role of BARDA in Vaccine Development
The Biomedical Advanced Research and Development Authority (BARDA) plays a pivotal role in fostering innovation in vaccine development, particularly during public health emergencies. By providing funding and resources, BARDA enables companies like Moderna to pursue groundbreaking research and expedite the development of critical vaccines. The recent $590 million investment is a testament to BARDA’s commitment to ensuring that the U.S. is prepared to face emerging infectious diseases through advanced vaccine technologies.
BARDA’s involvement is not limited to funding; it also includes collaboration in research and development, ensuring that the vaccines being produced are not only innovative but also practical for real-world application. This partnership between BARDA and Moderna exemplifies a proactive approach to public health preparedness, as it facilitates the swift identification and response to potential pandemic threats. As mRNA vaccines continue to evolve, the role of BARDA will remain crucial in guiding these efforts and ensuring effective outcomes.
Future Implications of mRNA Vaccines in Public Health
The future implications of mRNA vaccines extend far beyond their current applications in flu prevention. As researchers and public health officials explore the versatility of this technology, there is potential for mRNA vaccines to tackle a wide range of infectious diseases, including those caused by emerging pathogens. The support from HHS and substantial funding to Moderna signal a commitment to advancing public health initiatives that leverage innovative vaccine technologies.
The adaptability of mRNA vaccines could lead to the development of universal vaccines that provide broader protection against multiple strains of viruses. This capability would not only enhance pandemic preparedness but also potentially reduce the burden of seasonal flu outbreaks. As the landscape of infectious diseases continues to evolve, mRNA technology holds the promise of transforming vaccine development, ensuring that the public health infrastructure is equipped to manage future health challenges effectively.
Collaboration in Vaccine Research and Development
Collaboration is key to successful vaccine research and development, especially in the context of emerging infectious diseases. The partnership between Moderna and HHS, facilitated through BARDA, exemplifies how public and private sectors can work together to expedite vaccine innovation. By pooling resources and expertise, these collaborations create a robust framework for developing effective vaccines that can be deployed rapidly in response to health emergencies.
Such collaboration extends beyond financial support; it encompasses knowledge sharing, access to cutting-edge technology, and the integration of diverse research methodologies. By fostering an environment of cooperation, stakeholders can enhance the efficacy of vaccine development efforts, ensuring that the most promising candidates move swiftly through clinical trials and into the hands of healthcare providers. This collaborative approach is essential for building a resilient public health system capable of addressing future threats.
Global Impact of mRNA Vaccine Technology
The global impact of mRNA vaccine technology is profound, as it not only revolutionizes how vaccines are developed but also reshapes public health strategies worldwide. The ability to rapidly design and produce vaccines in response to emerging viruses is a game-changer for countries facing outbreaks. As seen during the COVID-19 pandemic, mRNA technology showcased its potential to provide timely solutions to urgent health crises, paving the way for its application in other infectious diseases like influenza.
Furthermore, the international collaboration surrounding mRNA vaccine development emphasizes the importance of a united global response to pandemics. As countries invest in their public health infrastructures and vaccine development capabilities, the lessons learned from mRNA technology could lead to more coordinated efforts in managing future health threats. The potential for widespread implementation of mRNA vaccines could significantly reduce the impact of pandemics, ultimately saving lives and improving health outcomes across the globe.
Frequently Asked Questions
What are mRNA vaccines and how do they work?
mRNA vaccines are a new type of vaccine that use messenger RNA to instruct cells to produce a protein that triggers an immune response. This technology, utilized by companies like Moderna, allows for faster vaccine development, particularly against emerging infectious diseases and pandemic threats.
How is HHS funding supporting the development of mRNA vaccines?
The HHS has awarded $590 million to Moderna to accelerate the development of mRNA vaccines targeting pandemic flu viruses, including H5N1 and H7N9 avian influenza. This funding aims to enhance pandemic preparedness and expand the clinical applications of mRNA technology.
What is the significance of Moderna’s mRNA flu vaccines in pandemic preparedness?
Moderna’s mRNA flu vaccines are crucial for pandemic preparedness as they can be rapidly developed and tailored to target specific flu strains. This capability enhances the response to potential influenza pandemics by ensuring faster and more effective vaccination strategies.
What progress has been made in the development of mRNA vaccines for H5N1 and H7N9?
Moderna has initiated phase 1/2 clinical trials for its investigational mRNA flu vaccine, targeting both H5 and H7 avian flu viruses. Positive initial trial results have set the stage for upcoming phase 3 studies, demonstrating the potential effectiveness of these mRNA vaccines.
How does mRNA technology improve vaccine development for emerging viruses?
mRNA technology improves vaccine development by enabling rapid response to emerging viruses, allowing for quicker modifications to vaccine formulations. This adaptability is essential in addressing new pandemic threats, as highlighted by the recent HHS funding for Moderna’s mRNA vaccine initiatives.
What are the potential benefits of mRNA vaccines over traditional vaccines?
mRNA vaccines offer several benefits over traditional vaccines, including faster development times, the ability to target multiple virus strains, and improved immune responses. The flexibility of mRNA technology allows for quick adjustments to address evolving infectious diseases.
How will the HHS funding influence future mRNA vaccine research?
The HHS funding will significantly influence future mRNA vaccine research by providing the necessary resources for Moderna to explore new vaccine candidates and conduct extensive clinical trials. This investment aims to enhance the United States’ readiness for future pandemics and strengthen vaccine technology.
What is the role of BARDA in mRNA vaccine development?
The Biomedical Advanced Research and Development Authority (BARDA) plays a pivotal role in mRNA vaccine development by providing funding and support for research and development efforts. BARDA’s involvement ensures that innovative vaccine technologies like mRNA are prioritized in public health preparedness strategies.
| Key Point | Details |
|---|---|
| Funding Amount | $590 million awarded to Moderna by HHS. |
| Purpose of Funding | To accelerate the development of mRNA vaccines against potential pandemic flu viruses. |
| Awarding Body | US Department of Health and Human Services (HHS). |
| Development Focus | Initial focus on H5N1 avian flu and H7N9 avian influenza. |
| Clinical Trials | Moderna is conducting phase 3 trials for H7N9 and has initiated phase 1/2 studies for mRNA-1018. |
| Previous Success | Initial trials have reported positive results for the mRNA vaccine candidates. |
| Strategic Importance | Investment aims to enhance US preparedness for flu pandemics and expand mRNA technology in vaccines. |
| Quotes | “mRNA technology will complement existing vaccine technology…” – Dawn O’Connell, former HHS Assistant Secretary. |
Summary
mRNA vaccines are at the forefront of innovative medical technology, particularly highlighted by the recent $590 million funding awarded to Moderna by the US Department of Health and Human Services. This significant investment aims to expedite the development of mRNA vaccines specifically targeting pandemic flu threats, showcasing the government’s commitment to enhancing public health preparedness. By leveraging mRNA technology, Moderna plans to advance its research on avian flu strains, ultimately aiming to protect against future pandemics. The positive initial trial results and the strategic development of multiple vaccine candidates underscore the potential of mRNA vaccines in combating infectious diseases.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.