The mRNA seasonal flu vaccine has become a topic of interest in recent years, particularly as advancements in mRNA technology promise to revolutionize how we combat influenza. French pharmaceutical giant Sanofi was at the forefront of this innovation, previously announcing plans to develop a next-generation mRNA flu vaccine. However, news broke recently that Sanofi has decided to halt its efforts in this area, shifting its focus instead toward a pandemic flu vaccine. While the company’s previous trials showed promise, especially against certain strains, they struggled with others, reinforcing the complexity of flu vaccine development. As the landscape of flu prevention evolves, Sanofi’s vaccine development strategies are pivotal to understanding the future of flu immunization and public health initiatives.
The recent halt in the development of next-gen influenza vaccines using messenger RNA technology has sparked discussions across the healthcare community. This cutting-edge approach aimed to offer improved protection against seasonal variants of the virus, yet faced challenges that led Sanofi to redirect its resources toward pandemic preparedness. With its high-dose and recombinant protein flu vaccines continuing to perform well, Sanofi’s strategy reflects a significant pivot in flu vaccine development. Seasonal flu vaccines are crucial in managing outbreaks, but the emergence of pandemic strains requires a different level of research and innovation. As companies like Sanofi navigate these waters, the implications for future influenza treatments remain a vital concern.
Sanofi’s Withdrawal from mRNA Seasonal Flu Vaccine Development
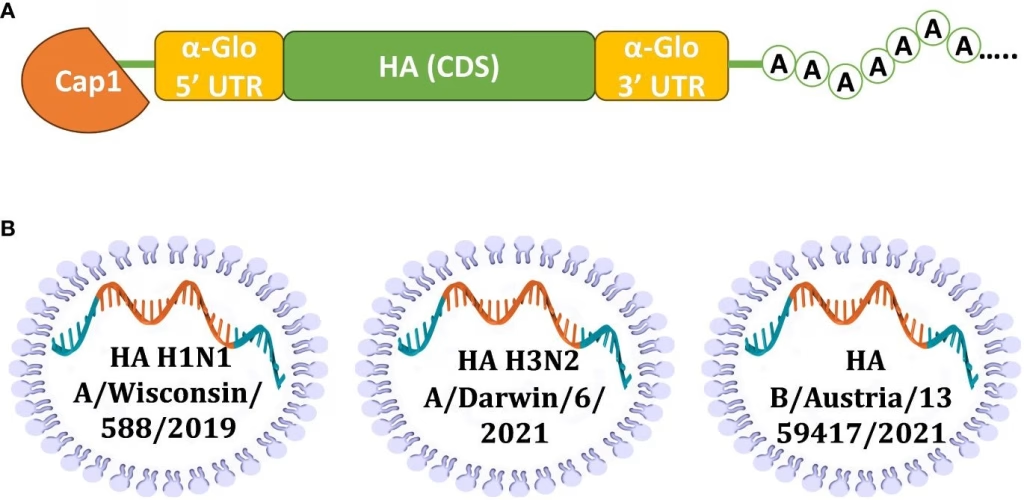
In a significant shift, Sanofi has decided to halt its plans for the development of an mRNA seasonal flu vaccine, citing challenges encountered during the clinical trial phase. Despite earlier ambitions to leverage mRNA technology for enhanced flu protection, particularly against B strains of the virus, the company recognized that the complex strain dynamics presented substantial hurdles. The decision underscores the unpredictability associated with vaccine development, especially with newer vaccine platforms that promise breakthrough efficacy but can also face unexpected setbacks in real-world applications.
The withdrawal from the mRNA seasonal flu vaccine project does not signal a retreat from flu vaccine innovation altogether. Instead, Sanofi is channeling its resources into a promising mRNA vaccine targeting pandemic strains, particularly H5 avian influenza. This strategic pivot highlights Sanofi’s commitment to maintaining a leadership role in vaccine technology, ensuring that their efforts align more closely with immediate public health needs and potential future outbreaks.
The Future of mRNA Technology in Vaccine Development
Sanofi’s experience with developing next-generation mRNA seasonal flu vaccines opens up a broader conversation about the future of mRNA technology in vaccine development. While the initial results against some strains were encouraging, the complexities of various influenza viruses illustrate the challenges faced by many pharmaceutical companies venturing into new vaccine territories. As seen with the mRNA technology used in COVID-19 vaccines, its potential is vast, yet it requires meticulous research and understanding of viral behavior to maximize effectiveness.
Despite the setback, Sanofi is not alone in exploring this promising technology. The continued investment in mRNA platforms is evident across the industry, with several companies actively pursuing mRNA solutions for multiple diseases. The success seen in combating COVID-19 with mRNA vaccines has bolstered confidence in this approach, which may eventually lead to more robust seasonal influenza vaccines that can withstand the rapid mutations of the virus. Future developments in this area may also integrate lessons learned from Sanofi’s initial trials, paving the way for more effective flu vaccines.
Advancements in Pandemic Flu Solutions
While Sanofi has stepped back from its plans regarding the mRNA seasonal flu vaccine, its ongoing research into a pandemic flu vaccine marks a critical step toward better preparedness for future outbreaks. With the successful outcomes of the current phase 1/2 studies focused on the H5 avian flu virus, Sanofi aims to establish a proactive defense against potentially devastating pandemic scenarios. This approach aligns well with the global emphasis on enhancing surveillance and vaccination strategies in anticipation of pandemic threats.
The ongoing studies and data evaluation for the H5 avian flu vaccine represent a significant move towards establishing a solid foundation for future public health responses. By focusing on innovative vaccine platforms, Sanofi aims to push the boundaries of conventional vaccine development, making strides toward vaccines that can not only effectively manage endemic strains but also respond rapidly to emerging pandemic threats. This level of commitment signals Sanofi’s intent to be at the forefront of vaccine development in the face of evolving viral challenges.
Sanofi’s Investment in mRNA Technology
Sanofi’s investment in mRNA technology marked a pivotal moment in its strategy to revolutionize vaccine development. The acquisition of Translate Bio in 2021 for $3.2 billion firmly established Sanofi’s commitment to harnessing the innovations of mRNA technology, which has demonstrated remarkable efficiency in responding to new viral challenges. However, the discontinuation of the mRNA seasonal flu vaccine development underscores the need to carefully assess both the potential and limitations of this technology in diverse viral landscapes.
This strategic investment indicates a long-term vision where mRNA technology could provide favorable outcomes in various vaccine applications, beyond seasonal flu prevention. Sanofi’s ongoing efforts in developing pandemic vaccines further showcase the versatility of mRNA approaches, with the hope that continued research will lead to enhanced protection against multiple influenza virus strains in one comprehensive solution.
Sanofi’s Commitment to Fighting Influenza
Despite recent hurdles in mRNA vaccine development, Sanofi remains committed to addressing influenza through its approved vaccines, such as Fluzone High-Dose and Flublok. These vaccines continue to provide robust protection against severe influenza outcomes, adding a layer of proven efficacy during flu seasons. The company’s focus on bolstering immunity through existing vaccines underscores a crucial aspect of public health, ensuring people have access to reliable prevention mechanisms while newer technologies are refined.
Sanofi’s strategy includes not only maintaining its portfolio of traditional vaccines but also identifying areas for improvement and innovation. Moving forward, Sanofi’s dual approach to harnessing past successes while exploring new technologies indicates a balanced method in tackling the challenges posed by influenza, ensuring that their contributions to public health are both immediate and forward-looking.
Challenges Faced by Next-Generation mRNA Flu Vaccines
The development of next-generation mRNA seasonal flu vaccines presents formidable challenges, particularly the variability of the influenza virus. One of the primary obstacles is the virus’s ability to mutate rapidly, which can drastically affect vaccine efficacy, especially for B strains. Sanofi’s initial promising results against A strains only highlight how complex the overall landscape of seasonal influenza can be, demonstrating that mRNA technology, while revolutionary, may still face limitations in adaptability.
Moreover, as seen in the recent developments, more extensive research and trials are essential to ensure any mRNA vaccine can adequately cover the spectrum of influenza strains encountered during flu seasons. Sanofi’s commitment to mRNA technology, in tandem with its ongoing innovations, aims to provide solutions, though the road ahead is laden with the need for continuous scientific evaluation and adaptation based on real-world effectiveness.
The Role of Sanofi in Vaccine Development News
Sanofi has been a critical player in vaccine development news, particularly reflecting the industry’s shift toward innovative technologies like mRNA. As the pharmaceutical landscape evolves, following Sanofi’s developments provides insights that extend beyond their products, illustrating broader trends in public health strategy and vaccine optimization. With their recent announcements, the company emphasizes how different vaccine technologies can be tailored to address specific viruses and pandemic threats.
Moreover, Sanofi’s advanced research into pandemic flu vaccine solutions sets a precedent for the industry, reinforcing the notion that preparedness is key in mitigating future health crises. Their comprehensive approach to vaccines—including maintaining existing products while also investigating new technologies—demonstrates a commitment to public health that resonates with ongoing global conversations around vaccination efficacy and innovation.
Exploring Future Vaccination Strategies with mRNA
The exploration of future vaccination strategies utilizing mRNA technology reflects an exciting yet complex frontier in immunology. Sanofi’s efforts, although marked by setbacks, also serve as a catalyst for further investigation into how mRNA can evolve to meet the challenges posed by emerging viral strains. Future strategies may involve collaborative approaches, integrating findings from various trial outcomes to create flu vaccines that offer broader, more enduring protection.
The mRNA platform holds the potential for rapid adaptation to mutations, making it an attractive option for future flu vaccine development. As more data becomes available through ongoing and future studies, the pharmaceutical industry, guided by the experiences of companies like Sanofi, may set new benchmarks in vaccination strategies, not only for seasonal flu but for potential pandemic threats as well. Enhancing existing frameworks while exploring new innovations will be vital in this endeavor.
The Importance of Evolving Vaccine Technologies
The importance of evolving vaccine technologies has never been more apparent than in the wake of recent public health challenges. Sanofi’s exploration of mRNA technology showcases the urgency and necessity of advancing vaccine platforms that can adapt quickly to changing virus dynamics. As traditional methods are coupled with cutting-edge technologies, the potential to offer superior protection and response capabilities increases, providing a crucial benefit in the realm of infectious disease management.
Evolving vaccine technologies can play a pivotal role in enhancing global health resilience, especially as the threat of pandemics looms. Companies like Sanofi, with their focus on innovative vaccine solutions, contribute significantly to public health discussions, advocating for a proactive rather than reactive approach to vaccination. By embracing both traditional and novel strategies, the industry can work collaboratively toward safeguarding populations worldwide.
Frequently Asked Questions
What is the Sanofi mRNA seasonal flu vaccine and why was its development canceled?
The Sanofi mRNA seasonal flu vaccine was a next-generation vaccine aimed at improving flu protection using mRNA technology. However, Sanofi announced the discontinuation of its development due to challenges in providing effective protection against certain flu strains, particularly B strains, despite promising results against A strains.
How does mRNA technology improve seasonal flu vaccines?
mRNA technology enhances seasonal flu vaccines by enabling a quicker response to emerging strains. This approach allows for precise targeting of the virus’s genetic information, which can lead to better immune response and potentially improved safety and efficacy compared to traditional flu vaccines.
What are the next steps for Sanofi after stopping the mRNA seasonal flu vaccine development?
Following the cancellation of the mRNA seasonal flu vaccine, Sanofi is focusing on its ongoing research for a pandemic flu vaccine based on an H5 avian flu platform. This development aims to prepare for future influenza pandemics with highly effective vaccine solutions.
Why is there a need for next-generation flu vaccines like the mRNA seasonal flu vaccine?
Next-generation flu vaccines, including mRNA seasonal flu vaccines, are needed to improve efficacy against a wider range of influenza strains, including emerging variants that could elude current vaccine protections. These innovations are crucial for enhancing public health responses to seasonal and pandemic flu.
What is the significance of Sanofi’s acquisition of Translate Bio in mRNA vaccine development?
Sanofi’s acquisition of Translate Bio in 2021 was significant as it bolstered the company’s capabilities in mRNA technology, paving the way for revolutionary approaches in vaccine development. This partnership aimed to advance research in next-generation vaccines, including pandemic responses, though the planned mRNA seasonal flu vaccine was ultimately discontinued.
What are the alternatives to the mRNA seasonal flu vaccine that Sanofi continues to support?
Despite discontinuing the mRNA seasonal flu vaccine, Sanofi continues to support and provide its high-dose Fluzone and recombinant Flublok vaccines, both of which are FDA-approved and have demonstrated strong protection against severe influenza outcomes.
What challenges did the Sanofi mRNA seasonal flu vaccine face during trials?
During trials, the Sanofi mRNA seasonal flu vaccine faced challenges in effectively combating B strains of the influenza virus, highlighting a common issue for first-generation mRNA flu vaccines. Despite initial success with A strains, the complexity of flu virus variants hindered the advancement of this vaccine.
How are pandemic flu vaccines different from seasonal flu vaccines?
Pandemic flu vaccines, like the one Sanofi is developing, are designed to target specific emerging strains that could lead to widespread outbreaks, while seasonal flu vaccines aim to protect against the most common circulating strains each flu season. The development strategies and technologies, such as mRNA, can differ significantly between the two types.
What are the implications of Sanofi focusing on pandemic flu vaccines instead of mRNA seasonal flu vaccines?
Sanofi’s shift towards pandemic flu vaccines implies a strategic realignment to prioritize vaccine solutions that address potentially catastrophic outbreaks. This may lead to advancements in vaccine technology, guiding future responses to public health threats, while the mRNA seasonal flu vaccine remains a concept for further exploration.
| Key Point | Details |
|---|---|
| Sanofi Stops mRNA Flu Vaccine Development | Sanofi has announced the halt of its next-generation mRNA seasonal flu vaccine development but is still working on a pandemic influenza vaccine. |
| Continued Focus on Pandemic Preparedness | The company is advancing a phase 1/2 study of an mRNA vaccine targeting H5 avian flu, with promising preliminary data. |
| Initial Progress with mRNA Seasonal Flu Vaccine | Sanofi previously reported encouraging results from trials but encountered challenges with B strains which affected further development. |
| Acquisition of Translate Bio | Sanofi invested heavily in mRNA technology by acquiring Translate Bio for $3.2 billion in 2021 to enhance its vaccine development capabilities. |
| Focus on Existing Vaccines | The company is prioritizing its Fluzone High-Dose and Flublok vaccines for protecting against influenza and its complications. |
Summary
The mRNA seasonal flu vaccine development faced challenges, leading Sanofi to discontinue its plans for this specific vaccine. While multi-strain protection remains a goal, the focus will shift to established vaccines and pandemic preparedness. Sanofi expresses commitment to continuing its innovation journey while balancing public health needs.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.