The latest MMRV vaccine recommendations announced by the Centers for Disease Control and Prevention (CDC) reflect significant shifts in childhood vaccination practices, driven by recent political and scientific discussions. In an effort led by Health and Human Services (HHS) Secretary Robert F. Kennedy Jr., these alterations aim to address skepticism surrounding vaccination policies, as well as the urgent need for higher vaccine uptake statistics among America’s youth. The MMRV vaccine, which combines protection against measles, mumps, rubella, and varicella, has been a topic of contention and debate amid childhood vaccination changes. These discussions raise crucial questions about the immunization schedule and potential health implications for children. As the CDC navigates these modifications, understanding the implications of the MMRV vaccine recommendations becomes essential for parents and public health advocates alike.
The recent updates to the combined vaccine for measles, mumps, rubella, and varicella, commonly known as chickenpox, indicate a pivotal moment in the realm of pediatric immunizations. This evolving landscape of vaccination strategies addresses rising concerns about vaccine hesitancy and overall protection against contagious diseases in young children. The discussions among health officials, including those from the Advisory Committee on Immunization Practices (ACIP), suggest a reevaluation of longstanding vaccination schedules amidst growing public discourse. With an eye on contemporary healthcare challenges, understanding these updated immunization guidelines is crucial for safeguarding future generations. This reexamination of childhood vaccine protocols underscores the ongoing tension between established medical recommendations and emerging public sentiment.
Understanding the MMRV Vaccine Recommendations
The recent modifications to the MMRV vaccine recommendations by the CDC’s Advisory Committee on Immunization Practices (ACIP) mark a significant shift in childhood vaccination policy. These changes have come amidst a broader debate regarding vaccine uptake in children and the efficacy of the MMRV vaccine itself, which protects against measles, mumps, rubella, and varicella (chickenpox). Historically, parents have been advised to administer separate vaccines for these ailments; however, the option for a combined MMRV vaccine remains available for those parents who choose to streamline the vaccination process. The committee’s decision underscores the need for ongoing dialogues about vaccination schedules and the potential implications of these changes on public health statistics surrounding childhood diseases.
It is essential to examine the impact these recommendations could have on vaccine uptake statistics in the coming years. With only about 15% of caregivers opting for the MMRV vaccine for their children aged 12 to 15 months, the ramifications of opting for separate vaccinations may lead to lower compliance with the vaccination schedule. Furthermore, concerns have been raised about the possibility of diseases such as measles and rubella re-emerging due to potential declines in vaccination rates. The ACIP Chair, Martin Kulldorff, acknowledging these fears, emphasized the importance of weighing the risks of febrile seizures against the significant benefits vaccinations provide in preventing serious illnesses.
Frequently Asked Questions
What are the recent changes to MMRV vaccine recommendations by the CDC?
The CDC’s Advisory Committee on Immunization Practices (ACIP) recently voted to modify MMRV vaccine recommendations, eliminating the requirement for separate measles, mumps, and rubella (MMR) and varicella vaccinations for children aged 12 to 47 months. However, the MMRV vaccine remains an option for parents of children in this age group.
How do the new MMRV vaccine recommendations impact childhood vaccination schedules?
The latest MMRV vaccine recommendations may impact childhood vaccination schedules by providing parents the option to choose the combined MMRV vaccine rather than separate vaccines. This change aims to streamline vaccine administration, although it raises concerns about potential decreases in overall vaccine uptake.
How does the MMRV vaccine relate to vaccine uptake statistics in children?
Currently, only about 15% of parents opt for the MMRV vaccine for children 12 to 15 months old. The CDC’s changes to MMRV vaccine recommendations could further influence these vaccine uptake statistics by altering parental choices regarding combined versus separate vaccinations.
What role did HHS Secretary Kennedy play in changing MMRV vaccine recommendations?
HHS Secretary Robert F. Kennedy Jr. has been influential in reshaping childhood vaccination policies. His recent modifications to MMRV vaccine recommendations reflect his ongoing efforts to challenge established vaccine guidelines, a move that has sparked considerable debate within the medical community.
Why is the MMRV vaccine important for preventing diseases like measles and mumps?
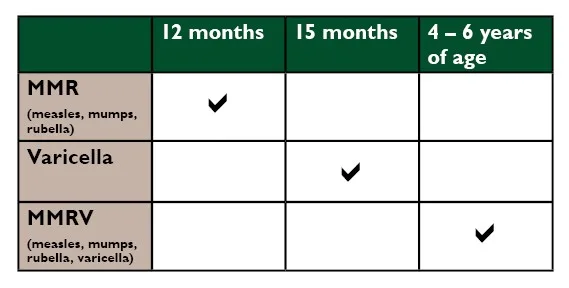
The MMRV vaccine is important because it helps protect children from serious diseases like measles, mumps, rubella, and varicella (chickenpox). The vaccine provides critical immunity, particularly during the vulnerable ages of 12 to 15 months and 4 to 6 years, helping to prevent potential outbreaks within communities.
What concerns have been raised regarding the new MMRV recommendations and children’s health?
Concerns about the new MMRV recommendations include the potential for a resurgence of diseases like measles and mumps if vaccination rates decline. Experts, including former CDC director Susan Monarez, have cautioned that changes led by Secretary Kennedy could jeopardize children’s health by increasing their risk of vaccine-preventable diseases.
How has the CDC’s approach to MMRV vaccine recommendations evolved over time?
The CDC last revisited MMRV vaccine recommendations in 2009, reaffirming its safety and efficacy. The recent changes to MMRV vaccine recommendations represent a significant shift as the ACIP discusses updated data and reflects on current public health needs amid a changing vaccine landscape.
What is the role of the Advisory Committee on Immunization Practices (ACIP) regarding MMRV vaccine recommendations?
The ACIP plays a critical role in evaluating and recommending immunization practices, including MMRV vaccine recommendations. This committee reviews safety and efficacy data and considers public health implications to guide vaccination schedules and recommendations for children.
Are there any risks associated with the MMRV vaccine according to recent discussions?
Yes, recent discussions have highlighted that the MMRV vaccine may carry a slightly higher risk of febrile seizures when given as the first dose compared to separate MMR and varicella vaccines. However, experts emphasize that these seizures are typically brief and do not result in long-term impacts.
What alternatives exist if parents do not wish to administer the MMRV vaccine?
For parents who are hesitant about the MMRV vaccine, alternatives include administering the individual measles, mumps, rubella (MMR) and varicella vaccines separately. The CDC continues to support vaccination for these diseases, and parents can consult with their healthcare providers to choose the best option for their children.
| Key Point | Details |
|---|---|
| CDC Modifications | CDC’s vaccine advisory group modified MMRV recommendations, amid ongoing challenges from HHS Secretary Robert F. Kennedy Jr. |
| Vaccination Schedule Changes | ACIP voted to stop recommending separate MMR and varicella vaccines for children 12 to 47 months but allows parents to choose MMRV. |
| Diverging State Recommendations | States are beginning to differ from CDC recommendations and insurance groups continue to support existing coverage based on established guidelines. |
| Concerns Over Safety | There are concerns regarding a higher risk of febrile seizures with MMRV compared to separate vaccinations. |
| Past Discussions | The ACIP last reviewed MMRV in 2009, confirming its efficacy and safety with an emphasis on discussions between doctors and parents. |
| Next Steps | Further votes on the hepatitis B vaccine and COVID-19 recommendations are scheduled. |
Summary
The recent discussions about MMRV vaccine recommendations highlight important changes in childhood vaccination policies. The CDC has altered its existing guidelines, with implications that could affect vaccine uptake rates and increase the risks of preventing diseases like measles and mumps. As parents and health officials navigate these modified recommendations, it remains crucial for families to consult healthcare professionals about the potential health implications and ensure their children are vaccinated appropriately against these preventable diseases.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.