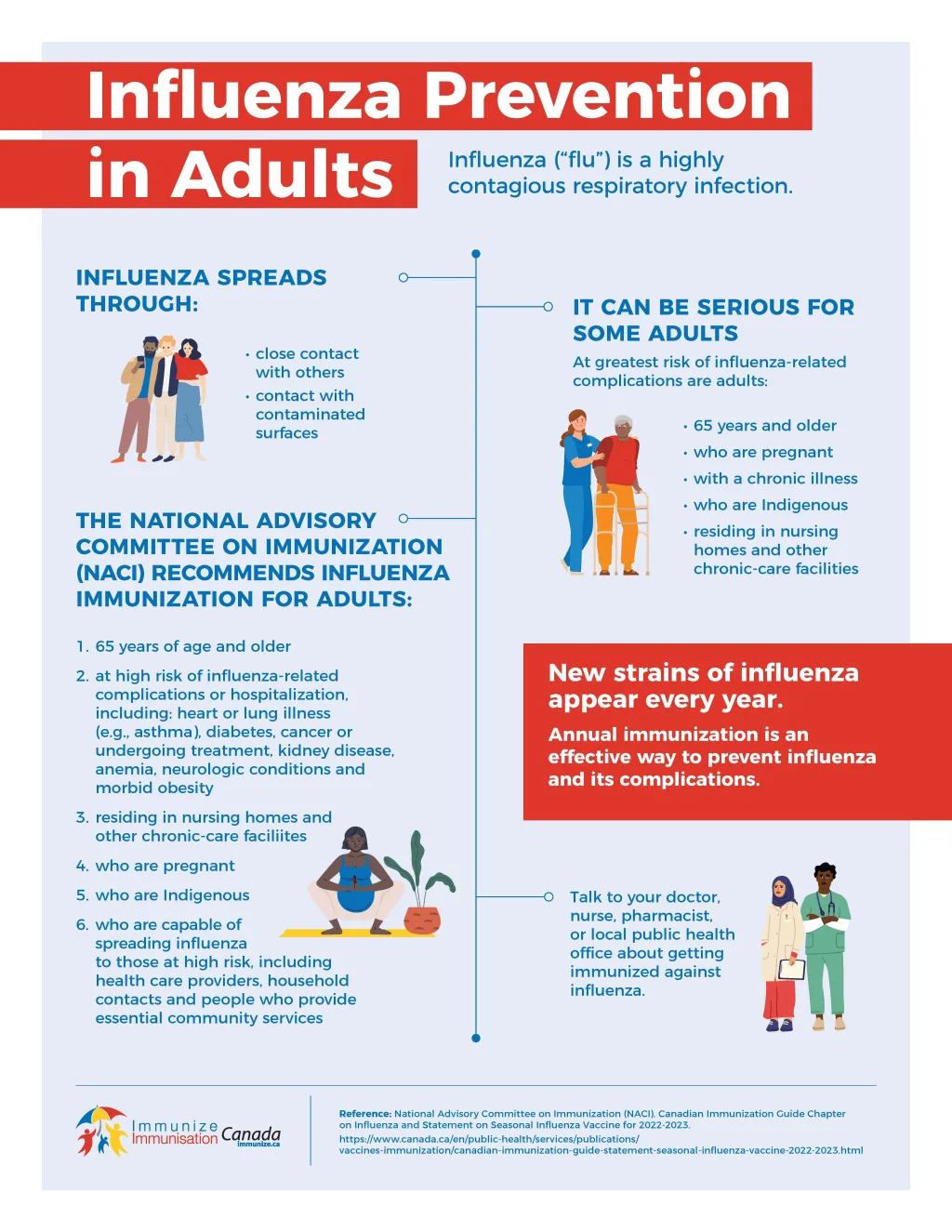
Influenza preventative measures are crucial for public health, especially with the emergence of new viral strains each season. Cidara Therapeutics has made significant strides in this area, recently securing up to $339 million from BARDA to support the development of CD388, a novel non-vaccine approach. This innovative therapy aims to provide a long-acting, universal solution against various strains of influenza, catering specifically to vulnerable populations. As highlighted by Cidara’s president and CEO, Jeffrey Stein, ensuring effective flu treatment options is vital for enhancing pandemic preparedness, particularly for those who are immunocompromised or elderly. The strategic partnership with BARDA will enhance domestic production capabilities for CD388, paving the way for broader flu protection across the United States.
In the realm of flu prevention, cutting-edge innovations like CD388 from Cidara Therapeutics are redefining our approach to combating influenza outbreaks. Rather than traditional vaccines, CD388 represents a next-generation therapeutic that is designed to offer protection without the need for immune activation. This type of approach could significantly change the landscape of flu treatment options, particularly for high-risk groups such as the elderly and those with underlying health conditions. The financial backing from BARDA underscores the critical importance of this development in achieving pandemic preparedness. With its unique mechanism, CD388 could very well set a new standard in managing seasonal and pandemic influenza.
The Importance of Influenza Preventatives in Pandemic Preparedness
Influenza remains a significant global health threat, particularly during peak seasons when outbreaks can overwhelm healthcare systems. Cidara Therapeutics’ innovative approach to developing CD388 highlights the urgent need for effective influenza preventatives in enhancing pandemic preparedness. This funding from BARDA is not merely a financial commitment but a recognition of the necessity for long-term solutions that transcend traditional vaccine limitations, especially for vulnerable populations such as the elderly and those with compromised immune systems.
In the face of potential pandemics, a universal influenza preventative like CD388 can provide a crucial line of defense. With the ability to offer protection against a variety of flu strains, this investigational drug has the potential to reduce seasonal flu-related illness and mortality significantly. The focus on developing such therapeutics indicates a shift in how healthcare providers prepare for and respond to influenza outbreaks, aiming to build robust defenses that do not rely solely on annual vaccination efforts.
How BARDA Funding Accelerates Development of CD388
The investment of up to 339 million dollars by BARDA marks a pivotal moment in flu treatment research. This funding will help accelerate the production and availability of CD388, a non-vaccine preventative that does not depend on immune response. The significance of this support cannot be overstated, as it ensures that Cidara Therapeutics can fast-track its development processes and bring new treatment options to market during critical public health circumstances.
This strategic partnership with BARDA highlights the government’s commitment to pandemic preparedness by investing in promising therapeutic alternatives. By supporting a drug that works as a long-acting small molecule inhibitor, BARDA is acknowledging the need for innovative solutions to combat influenza, particularly as traditional vaccine strategies may leave some populations inadequately protected. The future of flu treatment may see an exciting evolution thanks to such collaborations.
Understanding CD388: A New Hope for Influenza Treatment Options
CD388, an investigational drug-Fc conjugate developed by Cidara Therapeutics, presents a pioneering approach in the realm of influenza treatment options. Unlike existing vaccines that offer seasonal protection depending on immune response, CD388 is designed to provide consistent protection against multiple flu strains through a single administration. This innovation could revolutionize how we approach flu seasons, especially for individuals at higher risk of severe complications.
The differentiation from traditional vaccines lies in CD388’s mechanism of action. Since it is a low-molecular-weight biologic and not reliant on the immune response, it addresses a critical gap in current flu prevention strategies. For patients who may not respond robustly to vaccines, such as the elderly or immunocompromised individuals, CD388 provides a much-needed supplementary option in their preventative arsenal.
Potential Impact of CD388 on Seasonal Influenza
With its unique formulation, CD388 holds promise for significantly impacting seasonal influenza management. Data presented by Cidara indicates that CD388 may offer superior protection compared to traditional flu vaccines, particularly in populations that typically struggle with vaccine efficacy. This implications suggest a shift in treatment paradigms, where taunt flu seasons could see fewer hospitalizations and improved public health outcomes due to the availability of this innovative treatment.
Moreover, the potential for administration through simple methods—either subcutaneously or intramuscularly—could enhance patient compliance and accessibility. In areas where vaccination rates are typically low due to hesitancy or logistical issues, the introduction of an alternative therapeutic like CD388 could shift the dynamics of flu spread and management. As flu treatment options expand, the healthcare system could witness a profound decrease in the burden of flu, particularly during high-risk periods.
The Role of Cidara Therapeutics in Advancing Flu Prevention
Cidara Therapeutics is at the forefront of innovation in influenza prevention, positioning itself as a key player in developing solutions that directly address the limitations of traditional vaccines. The development of CD388 represents a significant advancement in flu treatment options, with the potential to offer broad protection against multiple strains of influenza. The company’s commitment to improving health outcomes for vulnerable populations underscores its role in shaping the future of pandemic preparedness.
By focusing on non-vaccine therapeutics, Cidara Therapeutics challenges the existing paradigms surrounding flu prevention and opens the door for new strategies that prioritize effectiveness and accessibility. As demonstrated by the collaboration with BARDA, the pharmaceutical company is aligning itself with national health priorities, fostering a collaborative approach to solving the complexities of influenza outbreaks and making substantial contributions to public health.
Benefits of Non-Vaccine Preventatives like CD388
Non-vaccine preventatives like CD388 offer a compelling alternative in the fight against influenza, particularly for individuals who may not achieve immunity through traditional vaccination. Since CD388 works independently of the immune system, it ensures that even those at high risk, such as the elderly and immunocompromised, can gain protection from various flu strains. This is a significant advancement in addressing the gaps left by current vaccine strategies.
Moreover, the broader implications of using long-acting, injectable therapeutics like CD388 could ease the logistical challenges associated with vaccine distribution and administration. As flu seasons tend to peak unpredictably, having a reliable preventative option that doesn’t rely on annual vaccination schedules could revolutionize public health responses, leading to lower influenza morbidity and mortality rates, especially in vulnerable populations.
CRAFTing a Comprehensive Future in Pandemic Preparedness
The initiative taken by Cidara Therapeutics, alongside BARDA, represents an essential leap towards comprehensive pandemic preparedness. CD388 is not just a treatment; it embodies a commitment to developing responsive public health solutions that can be deployed rapidly in the face of emerging threats. The ongoing evolution of viral pathogens necessitates the creation of versatile therapeutics that can adapt to the changing landscape of infectious diseases.
In light of recent global health challenges, the significance of innovative treatments like CD388 cannot be overstated. By prioritizing development that centers around efficacy irrespective of immune status, we pave the way for enhanced resilience against pandemics. This foresight in research is crucial, ensuring our health systems are better equipped to handle similar crises in the future, emphasizing the need for continuous investment and innovation in therapeutic options.
Dickensian Times: Perspectives on Flu Treatment Evolution
Reflecting on the evolution of influenza treatment, there is a stark contrast between past approaches and the promising future embodied by CD388. Traditional reliance on seasonal vaccines often leaves gaps in protection, particularly for those most susceptible to severe outcomes from the flu. The advent of non-vaccine options like CD388 offers a new era in treatment, where efficacy can be attained without the need for robust immune activation.
The traditional narratives of flu management often overlooked the complexities of individual health statuses. However, CD388 stands as a testament to the shift towards personalized healthcare solutions that recognize the diverse needs of populations. As researchers and healthcare providers work together to address these dynamics, we may witness not only an evolution in preventative strategies but also a significant reduction in the societal and economic burdens associated with influenza annually.
Future Directions in Flu Prevention and Treatment Research
As researchers continue to unveil the potential of therapeutics like CD388, it becomes clear that the landscape of flu prevention and treatment is on the cusp of transformative change. Future research will likely focus on optimizing the formulation and administration strategies for CD388, enhancing its efficacy and accessibility. Additionally, the ongoing evaluations of its effectiveness compared to traditional vaccines will provide critical insights into where it fits within the broader influenza management framework.
The integration of innovative therapeutics into flu prevention strategies highlights an exciting avenue for research that transcends the limitations of current methodologies. As understanding grows around drug mechanisms and patient responses, there is an opportunity to redefine seasonal influenza management, ultimately leading to greater public health resilience. This focus on multifaceted strategies will be instrumental in combating future influenza challenges more effectively.
Frequently Asked Questions
What is Cidara Therapeutics’ CD388 and how does it relate to influenza preventative measures?
CD388, developed by Cidara Therapeutics, is a novel non-vaccine preventative for influenza designed to provide long-lasting protection against various strains of flu. It works as a drug-Fc conjugate (DFC) that does not rely on immune system responses, making it a promising option for pandemic preparedness.
How does BARDA funding contribute to the development of influenza preventatives like CD388?
The Biomedical Advanced Research and Development Authority (BARDA) has committed up to $339 million to support the development of CD388, enabling Cidara Therapeutics to accelerate production and enhance domestic supply options for this innovative influenza preventative.
Can CD388 be used as a flu treatment option for immunocompromised individuals?
Yes, CD388 is particularly significant for treating immunocompromised individuals and older adults, as it does not depend on immune response for efficacy. This makes it an effective influenza preventative option for those at high risk for severe complications from flu infections.
What are the advantages of CD388 over traditional flu vaccines?
Unlike traditional flu vaccines, CD388 offers season-long protection against multiple flu strains with just one administration, either subcutaneously or intramuscularly. It has demonstrated effectiveness in protecting adults from influenza, potentially surpassing that of conventional flu vaccines.
How is CD388 developed to address pandemic preparedness?
CD388 is designed as a long-acting universal influenza preventative aimed at providing broad protection across all populations, crucial for pandemic preparedness. Its development responds to the need for effective solutions for those less responsive to vaccines, ensuring wider accessibility during flu outbreaks.
| Key Points |
|---|
| Cidara Therapeutics received $339 million from BARDA for CD388 development. |
| CD388 is a non-vaccine preventative for seasonal and pandemic influenza. |
| CD388 provides long-lasting protection against all influenza strains. |
| The drug works independently of the immune response, making it effective for all populations. |
| CD388 can be administered via subcutaneous or intramuscular injection. |
| Evidence suggests CD388 may be more effective than traditional flu vaccines. |
Summary
Influenza preventative strategies are crucial for safeguarding public health, particularly for vulnerable populations. Cidara Therapeutics has made significant progress in developing CD388, a novel non-vaccine approach to prevent influenza, which could enhance pandemic preparedness and protection for individuals who are immunocompromised or elderly. With a funding boost of $339 million from BARDA, this innovative treatment promises to provide long-lasting protection against multiple strains of the virus without relying on the immune response, presenting a compelling alternative to traditional vaccines.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.