DoxyPEP, or doxycycline post-exposure prophylaxis, is gaining attention as an innovative strategy for sexually transmitted infection (STI) prevention, particularly among high-risk populations. Recently released guidance from the European Centre for Disease Prevention and Control (ECDC) advocates for its cautious use in supporting sexual health strategies, aiming to reduce the incidence of STIs like chlamydia and syphilis. While doxyPEP shows promise in combating the rising rates of these infections, the ECDC emphasizes the importance of monitoring its implementation to prevent the rise of antimicrobial resistance (AMR). This guidance underscores that the administration of doxyPEP should be tailored to individual clinical assessments, reflecting the complexities surrounding STI prevention measures. By considering both the potential benefits and risks involved, doxyPEP represents a crucial component of a holistic approach to sexual health strategy in Europe and beyond.
Doxycycline post-exposure prophylaxis (doxyPEP) is becoming a key player in strategies aimed at reducing sexually transmitted diseases (STDs) among vulnerable groups. The recent recommendations from European health authorities highlight doxyPEP’s role in STI-related health initiatives, particularly in addressing the alarming uptick in cases of infections like syphilis and chlamydia. Emphasizing individual risk assessments, these guidelines advocate for a vigilant approach to implementing this preventive measure, in light of growing concerns about the development of antibiotic resistance. By framing doxyPEP within a broader context of sexual health planning, public health officials hope to create effective solutions while safeguarding against potential counterproductive outcomes of increased AMR. The introduction of this prophylactic method marks a significant shift in the understanding of STIs and paves the way for informed, responsible public health policy.
ECDC Guidance on DoxyPEP Implementation
The European Centre for Disease Prevention and Control (ECDC) has issued guidance regarding the cautious use of doxycycline post-exposure prophylaxis (doxyPEP) for preventing sexually transmitted infections (STIs). This guidance acknowledges the increasing incidence of STIs, particularly syphilis, which has risen alarmingly among high-risk populations. By recommending doxyPEP under specific circumstances, the ECDC highlights the importance of clinical judgment and the necessity for careful monitoring to prevent adverse public health outcomes such as antimicrobial resistance (AMR). Public health officials are thus urged to consider a comprehensive sexual health strategy that includes regular STI testing and other preventive measures alongside doxyPEP.
Despite recognizing the benefits of doxyPEP in clinical trials, the ECDC’s guidance refrains from offering a blanket endorsement. Instead, they emphasize a tailored approach where healthcare providers assess each individual’s risk level before recommending doxyPEP. This individual-centered care is critical, particularly given the rising instances of resistant bacterial strains seen in Europe. The guidance serves as a reminder that while doxyPEP can reduce STI incidences, it is not a standalone solution; it should be integrated within broader sexual health strategies including vaccination and education about safe sex practices.
Frequently Asked Questions
What is doxyPEP and how does it relate to STI prevention?
DoxyPEP, short for doxycycline post-exposure prophylaxis, is an antibiotic intervention recommended for sexually transmitted infection (STI) prevention. It involves administering doxycycline within 24 hours after unprotected sex, particularly for high-risk individuals such as men who have sex with men (MSM) and transgender women, to significantly reduce incidence rates of infections like chlamydia and syphilis.
What guidance has the ECDC provided regarding doxyPEP?
The European Centre for Disease Prevention and Control (ECDC) has issued cautious guidance on doxyPEP usage for STI prevention. While recognizing its effectiveness in high-risk groups, the ECDC emphasizes that decisions to use doxyPEP should rely on individual clinical assessments, not population-level interventions. They also stress the importance of integrating doxyPEP into a comprehensive sexual health strategy, along with regular STI testing and treatment.
What are the risks associated with the widespread use of doxyPEP?
Concerns around doxyPEP include the potential for increasing antimicrobial resistance (AMR), particularly regarding Neisseria gonorrhoeae strains that are becoming resistant to tetracycline, the active ingredient in doxycycline. The ECDC has highlighted risks associated with the emergence of mobile resistance genes that may complicate future bacterial infections, posing threats not just to doxyPEP users but to the broader population.
How does doxyPEP fit into a sexual health strategy?
DoxyPEP should be viewed as part of a comprehensive sexual health strategy. This includes routine STI screening, vaccination, HIV prevention measures, and partner notification services. The ECDC underscores that doxyPEP is not a standalone answer but should complement other preventive health measures to be effective in reducing STI rates.
What is the ECDC’s stance on doxyPEP for preventing gonorrhea?
The ECDC advises against using doxyPEP as an effective preventative measure for gonorrhea due to existing high levels of tetracycline resistance. With nearly 62.3% of gonorrhea strains in Europe resistant to tetracycline, the organization is cautious about promoting doxyPEP for this particular STI, focusing instead on its use for preventing syphilis.
How should doxyPEP be monitored to ensure safety and effectiveness?
Monitoring the use of doxyPEP is crucial. The ECDC recommends that health authorities maintain oversight of its application, especially regarding its effects on antimicrobial resistance and public health. Regular assessments and data collection on doxyPEP users, as well as adherence to national guidelines, will help evaluate the intervention’s impact and safety.
What have studies shown about the effectiveness of doxyPEP?
Clinical trials and real-world studies indicate that doxyPEP significantly lowers the incidence of chlamydia and syphilis among high-risk groups, particularly MSM and transgender women. These findings have led to its recommendation by health officials in several countries, though the ECDC emphasizes a targeted application considering individual risk factors.
What does the ECDC suggest regarding doxyPEP implementation at the national level?
The ECDC’s guidance recommends that countries consider implementing doxyPEP with caution, prioritizing high-risk groups and involving clinical judgment in decision-making. The agency advocates for individual assessments rather than broad public health interventions and highlights the need for ongoing monitoring regarding antimicrobial resistance.
| Key Point | Details |
|---|---|
| ECDC Guidance | The ECDC released guidance for doxyPEP use for high-risk individuals and emphasized the importance of clinical judgement and national guidelines. |
| DoxyPEP Effectiveness | Clinical trials show doxyPEP is effective in reducing STIs like chlamydia and syphilis in men who have sex with men (MSM) and transgender women. |
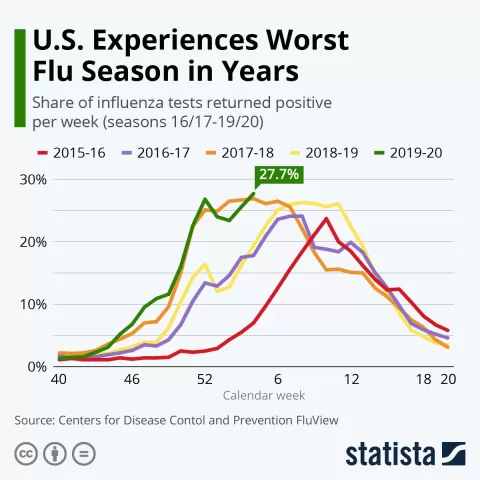
| Target Population | Focus on high-risk groups, particularly for syphilis, which has seen a 53% increase in EU/EEA countries since 2019. |
| Antimicrobial Resistance Concerns | Concerns about increased tetracycline resistance, particularly in Neisseria gonorrhoeae, make wide implementation risky. |
| Individual Assessment | Decisions on doxyPEP use should be based on individual clinical assessments of risk. |
| Monitoring usage | Ongoing monitoring of doxyPEP use and its public health impact is necessary to mitigate risks of AMR. |
| Non-Binding Recommendations | ECDC’s recommendations guide countries on assessing doxyPEP policy without enforcing mandatory applications. |
Summary
doxyPEP, or doxycycline post-exposure prophylaxis, has been a focal point in the fight against sexually transmitted infections (STIs) according to the latest ECDC guidance. While the ECDC recognizes its potential effectiveness, especially among high-risk groups like MSM and transgender women, it emphasizes caution due to significant concerns regarding antimicrobial resistance. Monitoring and tailored individual assessments are crucial to integrate doxyPEP within a broader sexual health strategy, ensuring that both public health and individual considerations are addressed. The implementation of this proactive approach must align with sound clinical judgement and national health guidelines to safeguard against rising bacterial resistance.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.