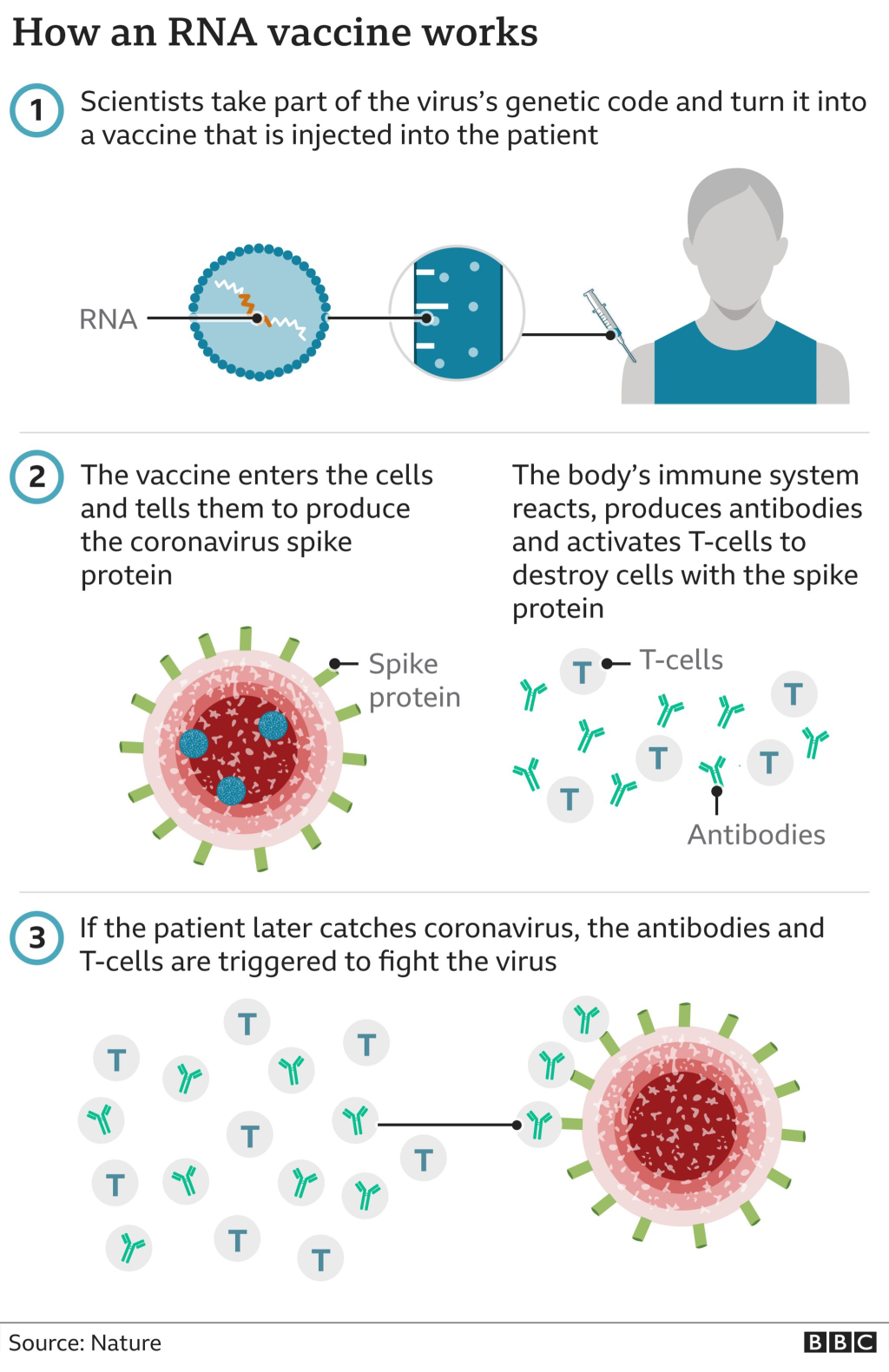
RNA vaccines have emerged as a groundbreaking innovation in the field of biotechnology, revolutionizing the way we approach vaccination. Recently, the Coalition for Epidemic Preparedness Innovations (CEPI) announced a significant $5 million funding award to Ethris, a German company dedicated to advancing next-generation RNA vaccines. This funding will facilitate the development of spray-dried RNA vaccines, a method that promises stability at room temperature and the ability for nasal delivery. By incorporating spray-drying technology, akin to methods used in asthma inhalers, these vaccines aim to provide a more accessible option for widespread immunization. With the focus on enhancing vaccine accessibility and improving delivery systems, RNA vaccines are poised to play a pivotal role in pandemic preparedness and response.
In the realm of immunization, messenger RNA (mRNA) vaccines represent a cutting-edge approach, leveraging biotechnology to combat infectious diseases. Recent initiatives, such as the funding from the Coalition for Epidemic Preparedness Innovations (CEPI), aim to propel the development of innovative delivery methods, including nasal administration and spray-dried formulations. This advancement can significantly improve vaccine accessibility, particularly in regions with limited resources, by eliminating the need for cold-chain logistics. Such developments not only enhance the practicality of vaccines but also align with the growing demand for needle-free options, addressing concerns like needle phobia. Overall, the evolution of RNA-based vaccines signifies a transformative step in global health initiatives.
The Future of RNA Vaccines in Global Health
RNA vaccines represent a groundbreaking advancement in the field of immunization, particularly in combating infectious diseases. The recent funding awarded to Ethris by the Coalition for Epidemic Preparedness Innovations (CEPI) highlights the potential for these vaccines to revolutionize public health strategies. Incorporating innovative methods like spray-drying not only enhances the stability of RNA vaccines but also expands their usability in real-world scenarios, especially in regions with limited healthcare infrastructure.
Moreover, as RNA technology continues to evolve, the focus will shift towards ensuring that vaccines are not only effective but also accessible to populations worldwide. This is crucial in the face of emerging infectious threats, as the rapid deployment of RNA vaccines can significantly mitigate viral transmission. CEPI’s investment in Ethris underscores a commitment to fostering biotechnology innovation that prioritizes both efficacy and accessibility in vaccine distribution.
The Role of Spray-Dried RNA Vaccines in Vaccine Accessibility
Spray-dried RNA vaccines are poised to enhance vaccine accessibility drastically, particularly in low-resource settings. The technology behind spray-drying allows for creating stable vaccine formulations that do not require refrigeration, addressing one of the most significant barriers in vaccine distribution. This could lead to broader immunization coverage, especially in remote areas where cold-chain logistics can be challenging.
Additionally, the nasal delivery mechanism of these vaccines presents a needle-free alternative that can alleviate fears associated with traditional injections. This approach could potentially increase vaccine uptake among individuals who experience needle phobia, further expanding the reach of immunization efforts. By leveraging CEPI’s funding to refine this technology, Ethris is at the forefront of a movement that seeks to make RNA vaccines more user-friendly and accessible to all.
CEPI Funding and Its Impact on Biotechnology Innovation
The Coalition for Epidemic Preparedness Innovations (CEPI) plays a pivotal role in funding innovative projects aimed at enhancing global health security. The recent $5 million funding award to Ethris is a testament to CEPI’s commitment to supporting biotechnology innovation that can address pressing public health challenges. By investing in the development of spray-dried RNA vaccines, CEPI is enabling researchers to explore new frontiers in vaccine delivery and formulation.
This funding is not merely financial support; it symbolizes a strategic move towards fostering a diverse portfolio of vaccine technologies that can respond rapidly to outbreaks. By backing projects that prioritize stability and ease of delivery, such as those focused on nasal administration, CEPI is paving the way for the next generation of vaccines that could be deployed effectively in varied environments across the globe.
Nasal Delivery Vaccines: A Game Changer in Immunization
Nasal delivery vaccines are emerging as a revolutionary approach to immunization, particularly for RNA vaccines. This method offers a non-invasive alternative to traditional injections, making it more appealing for populations resistant to needles. The recent advancements in spray-drying technology allow for these vaccines to be administered easily while maintaining their stability and effectiveness, a significant leap forward in vaccine delivery systems.
Furthermore, the potential for nasal delivery to induce mucosal immunity is a critical factor in limiting virus transmission. By targeting the mucosal surfaces directly, these vaccines could provide a more robust immune response, thereby enhancing overall efficacy. The integration of this technology into RNA vaccines could change the landscape of public health, especially in the face of respiratory infections.
Enhancing Thermostability in RNA Vaccines
Thermostability is a crucial characteristic for vaccines, particularly for those intended for deployment in resource-limited settings. The innovative spray-drying technique being developed for RNA vaccines by Ethris aims to address this challenge by producing formulations that can withstand higher temperatures without losing potency. This advancement is particularly relevant for countries where maintaining cold-chain logistics is not feasible.
By enhancing the thermostability of RNA vaccines, the potential for widespread distribution increases significantly. This not only facilitates easier access but also reduces the logistical burdens associated with vaccine transport and storage. As the global health community continues to seek solutions for equitable vaccine access, innovations that improve stability and usability will be vital.
Transforming Vaccine Delivery with Biotechnology
The integration of cutting-edge biotechnology in vaccine development is transforming how immunizations are delivered to populations worldwide. Ethris, through its pioneering work on spray-dried RNA vaccines, exemplifies how innovative approaches can streamline vaccine administration and enhance public health responses. These advancements are critical in preparing for future pandemics, as they enable rapid production and distribution of vaccines.
Moreover, the emphasis on biotechnology innovation aligns with global health initiatives aimed at improving vaccine accessibility. By focusing on methods that reduce the need for cold-storage and needle-based delivery, researchers are working towards solutions that meet the unique needs of various populations. The ongoing support from organizations like CEPI is essential in fostering these developments, ensuring that transformative technologies reach those who need them the most.
Addressing Vaccine Hesitancy through Innovative Delivery Methods
Vaccine hesitancy remains a significant challenge in achieving widespread immunization. Innovative delivery methods, such as nasal spray vaccines, have the potential to address some of the concerns associated with traditional injectables. By providing a needle-free option, these vaccines can alleviate fears and increase acceptance among individuals who may be reluctant to receive vaccinations due to needle phobia.
Moreover, the ease of administration associated with nasal delivery can facilitate more extensive vaccination campaigns, particularly in schools and community centers. As public health officials strive to ensure high vaccination rates, leveraging new technologies like spray-dried RNA vaccines can be a crucial strategy in overcoming barriers to immunization acceptance.
The Impact of RNA Vaccine Technology on Pandemic Preparedness
The COVID-19 pandemic has underscored the urgent need for rapid vaccine development and deployment. RNA vaccines, with their ability to be designed and manufactured swiftly, play an essential role in pandemic preparedness. The funding awarded to Ethris by CEPI for the development of spray-dried RNA vaccines is a forward-thinking response to the lessons learned from recent global health crises.
By investing in technologies that enhance the speed and accessibility of vaccine delivery, the global health community is better equipped to respond to future outbreaks. The innovations in RNA vaccine technology not only promise to improve response times but also ensure that vaccines can reach populations in diverse settings, ultimately protecting public health more effectively.
Building a Sustainable Supply Chain for RNA Vaccines
Establishing a sustainable supply chain for RNA vaccines is crucial in ensuring that these innovations reach those who need them most. The spray-drying technology being explored by Ethris could revolutionize the storage and transportation of vaccines, eliminating the dependence on cold-chain logistics. This shift would enable easier distribution, especially to remote and underserved areas.
In addition, a more sustainable supply chain can contribute to reducing the overall costs associated with vaccine distribution. By decreasing the need for refrigeration and complex logistics, resources can be redirected towards increasing production capacity and improving healthcare infrastructure. As CEPI supports such initiatives, the focus on sustainability will be vital in achieving global vaccination goals.
Frequently Asked Questions
What are RNA vaccines and how do they work?
RNA vaccines utilize messenger RNA (mRNA) to instruct cells in the body to produce a protein that triggers an immune response. This innovative biotechnology approach enables the rapid development of vaccines against infectious diseases by teaching the immune system to recognize and combat pathogens.
How is CEPI funding advancing the development of RNA vaccines?
CEPI funding is crucial for advancing RNA vaccines as it supports research and development initiatives. Recently, CEPI awarded $5 million to Ethris to develop spray-dried RNA vaccines, enhancing their stability and accessibility, particularly for nasal delivery.
What are the benefits of spray-dried RNA vaccines?
Spray-dried RNA vaccines offer significant benefits, including enhanced stability at room temperature, which eliminates cold-chain storage requirements. This makes them more accessible, especially in low-resource settings, and allows for easier administration through nasal delivery.
How does nasal delivery improve RNA vaccine accessibility?
Nasal delivery of RNA vaccines enhances accessibility by providing a needle-free option, which can alleviate concerns for those with needle phobia. This method also aims to achieve mucosal immunity, potentially reducing virus transmission and improving overall vaccine uptake.
What role does biotechnology innovation play in the development of RNA vaccines?
Biotechnology innovation is vital in developing RNA vaccines, as it drives advancements in vaccine formulation, stability, and delivery methods. Innovations like spray-drying technology can transform RNA vaccines into more practical solutions for global health challenges.
Why is vaccine accessibility important for RNA vaccines?
Vaccine accessibility is critical for RNA vaccines to ensure that all populations, particularly in low-resource countries, can receive timely immunization. By improving stability and delivery methods, such as through spray-dried formulations, RNA vaccines can reach a broader audience.
What challenges do traditional RNA vaccines face regarding storage?
Traditional RNA vaccines typically require cold storage throughout the supply chain, making distribution logistically challenging, especially in low-resource countries. The development of spray-dried RNA vaccines aims to address this challenge by ensuring stability at room temperature.
Can spray-dried RNA vaccines be a game changer for global vaccination efforts?
Yes, spray-dried RNA vaccines have the potential to be a game changer for global vaccination efforts by improving stability, simplifying administration, and enhancing accessibility, ultimately increasing immunization rates worldwide.
| Key Point | Details |
|---|---|
| Funding Announcement | CEPI announced a $5 million award to Ethris for RNA vaccine development. |
| Vaccine Type | Development of spray-dried RNA vaccines suitable for nasal delivery. |
| Technology Used | Uses spray-drying technology to produce stable powders for nasal administration. |
| Advantages | Enhances practicality and accessibility, eliminates cold-chain storage needs. |
| Mucosal Immunity | Nasal administration may help achieve mucosal immunity, reducing virus transmission. |
| Impact on Global Health | Could transform vaccine delivery, especially in low-resource countries. |
Summary
RNA vaccines represent a groundbreaking advancement in vaccine technology, particularly with the recent announcement from CEPI regarding their support for Ethris’s development of spray-dried RNA vaccines. This innovative approach aims to create vaccines that are not only stable at room temperature but also suitable for nasal delivery. The potential to eliminate cold-chain storage requirements makes RNA vaccines more accessible, especially in resource-limited settings. By addressing logistical challenges and enhancing ease of administration, RNA vaccines could significantly improve global vaccination efforts.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.