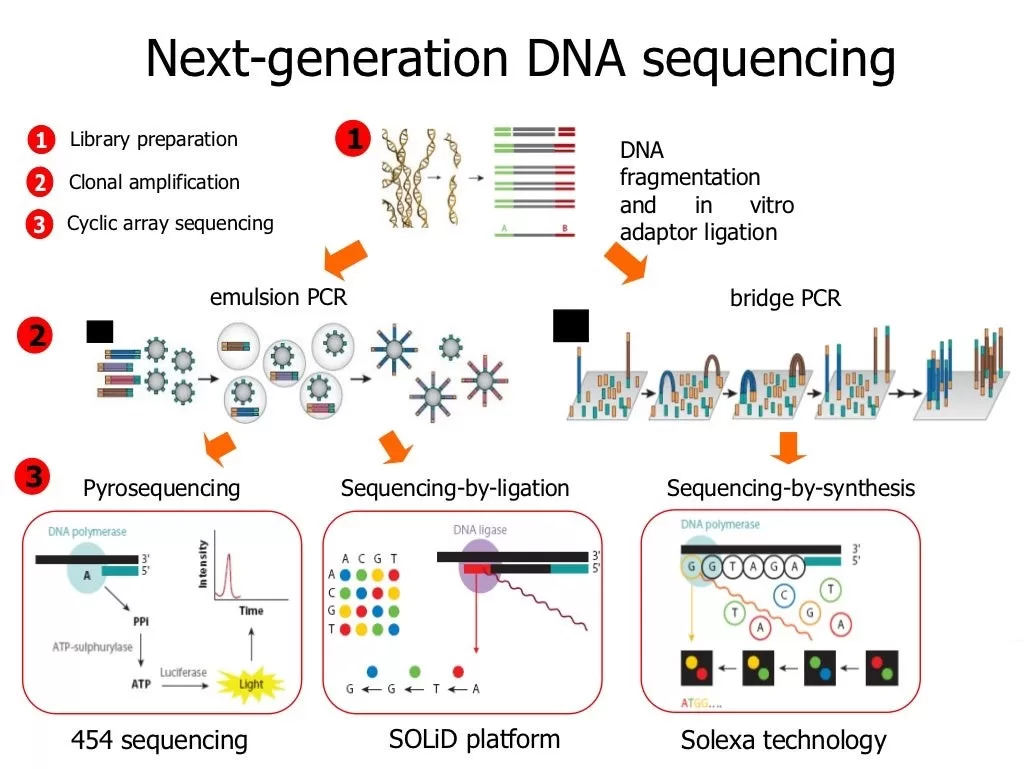
Next-Generation Sequencing (NGS) is at the forefront of transforming modern genomics, offering unprecedented insights into genetic data through high-throughput methodologies. The NGS Quality Initiative plays a crucial role in overcoming the clinical laboratory challenges that arise during the implementation of this advanced technology, particularly in public health laboratories. As the complexity of NGS increases with the introduction of diverse platforms and bioinformatics advancements, ensuring quality and consistency has become paramount. This initiative aims to streamline NGS method validation processes, providing essential resources for laboratories to navigate regulatory requirements effectively. By fostering innovation and collaboration, the NGS Quality Initiative is paving the way for a more reliable and efficient use of sequencing technologies in both clinical and research settings.
Revolutionizing our understanding of genetics, next-gen sequencing technologies have made incredible strides in recent years, significantly enhancing our ability to analyze large datasets. With the focus on the NGS Quality Initiative, laboratories are equipped to confront prevalent challenges related to personnel retention and method validation. These platforms are increasingly indispensable in public health contexts, where accurate and efficient diagnostics are paramount. Through a comprehensive approach that includes bioinformatics advancements, the initiative supports laboratories in maintaining compliance with ever-evolving standards. Ultimately, these efforts ensure that the deployment of advanced sequencing methods benefits not only research but also improves clinical outcomes.
Understanding the NGS Quality Initiative
The Next-Generation Sequencing (NGS) Quality Initiative is a pioneering effort led by renowned organizations including the CDC and the Association of Public Health Laboratories (APHL), which seeks to combat the myriad challenges faced by clinical and public health laboratories. This initiative focuses on developing comprehensive tools and resources aimed at establishing a robust quality management system that can efficiently navigate complex regulatory requirements and enhance the overall quality of NGS implementations. By addressing these critical aspects, the initiative plays an essential role in operationalizing NGS technologies in varied laboratory settings, ensuring that public health and clinical laboratories can adequately meet evolving standards.
Through the deployment of resources such as the QMS Assessment Tool and NGS Method Validation Plan, the initiative provides vital guidance for laboratories seeking to implement NGS technology successfully. These tools are designed not only to aid in method validation but also to support laboratories in addressing broader quality control challenges. Furthermore, by promoting best practices and supporting educational efforts, the NGS Quality Initiative empowers clinical laboratory staff to enhance their skills and ensure their readiness in adapting to new technologies and regulatory frameworks.
Challenges in Clinical and Public Health Laboratories
The integration of Next-Generation Sequencing into clinical and public health laboratories is accompanied by a unique set of challenges. One significant obstacle is the need for specialized personnel who possess the skills and knowledge to handle the complexities of NGS workflows. According to a recent survey conducted by the APHL, approximately 30% of public health laboratory staff expressed intentions to leave the workforce, signaling potential difficulties in maintaining a skilled workforce capable of producing high-quality sequencing results. This situation raises concerns on how labs will continue to meet stringent quality standards that are vital for public health.
Moreover, the challenges extend to equipment and process management, as laboratories must navigate the intricacies of diverse NGS platforms and the associated validation processes. Ensuring compliance with regulatory requirements can be resource-intensive; thus, laboratories must prioritize strategically using innovative resources provided by initiatives like the NGS Quality Initiative. At the same time, this underscores the importance of continuous training and competency evaluation tools, which are essential for keeping personnel engaged and well-prepared to implement the latest technological advancements in bioinformatics and laboratory practices.
Advancements in Bioinformatics and NGS
The field of bioinformatics has seen remarkable advancements that significantly enhance the capabilities and applications of Next-Generation Sequencing. Innovations in data analysis and computational methodologies have facilitated the development of more precise and efficient algorithms for interpreting NGS data. With these advancements, laboratories can expect improved accuracy in pathogen detection and the ability to process vast amounts of genomic data in a shorter time frame. Such improvements are particularly relevant in addressing public health crises, where rapid and accurate sequencing can inform timely responses to emerging infectious diseases.
However, the integration of these bioinformatics advancements into laboratory practices is not without its challenges. For instance, laboratories must ensure that they validate their bioinformatics pipelines alongside their wet lab processes, which can be demanding in terms of time and resources. The NGS Quality Initiative recognizes this complexity and aims to provide resources that assist laboratories in navigating both the laboratory and computational aspects of NGS method validation. By fostering a more holistic approach to quality assurance, the initiative seeks to enhance the reliability of NGS technologies in clinical applications.
The Role of NGS Method Validation
NGS Method Validation is a critical component of ensuring reliable outcomes in sequencing protocols. The complexity of various sample types and the advent of target and agnostic sequencing techniques pose considerable challenges in the validation process. Laboratories must establish rigorous validation protocols that account for these variables while adhering to industry standards and regulations. The NGS Quality Initiative addresses these needs by providing structured frameworks and resources that guide laboratories through the intricacies of method validation and revalidation procedures.
Additionally, the emphasis on comprehensive method validation is essential not only for academic and research purposes but also for clinical applications where accurate results can directly impact patient care. By implementing the guidelines and tools developed by the NGS Quality Initiative, laboratories can minimize variability and ensure consistent results across different platforms and applications. This assurance of quality is crucial for maintaining public trust and collaboration in the ever-evolving landscape of genomic medicine.
Overcoming Workforce Challenges
The retention of skilled personnel in clinical and public health laboratories is a growing concern, particularly with the rapid adoption of Next-Generation Sequencing technologies. As emphasized by the findings from the APHL survey, a significant portion of laboratory staff is contemplating exiting the workforce, highlighting the need for strategic efforts to address this impending talent gap. To mitigate this challenge, the NGS Quality Initiative has developed a series of training programs and competency evaluations designed to enhance workforce development and retention.
These training initiatives not only deliver essential knowledge about NGS methodologies but also aim to foster a culture of continuous professional development within laboratories. By investing in their workforce and ensuring they have access to the latest advancements in NGS technologies and bioinformatics, laboratories can build a more resilient and adaptable team. This empowerment enables laboratories to maintain high-quality standards and effectively address the diverse challenges that accompany NGS implementations in a public health context.
Impact of Regulatory Standards on NGS Implementation
The implementation of Next-Generation Sequencing technologies in clinical and public health settings must align with strict regulatory standards. Compliance with these standards is vital for ensuring the reliability of sequencing results, which can greatly influence public health responses. The NGS Quality Initiative recognizes the importance of navigating these regulatory frameworks, providing invaluable tools to help laboratories maintain compliance while implementing NGS methodologies. This support is essential in fostering confidence in NGS as a legitimate tool for disease diagnosis and monitoring.
Challenges related to regulatory compliance often arise from the dynamic nature of NGS advancements, requiring laboratories to constantly adapt their protocols and validation methods. As bioinformatics continues to shape NGS applications, staying informed about current regulations and establishing tailored validation practices becomes crucial. The guidance offered through initiatives like the NGS Quality Initiative equips laboratories with the necessary resources to navigate these evolving standards effectively, thus enhancing the integrity of NGS technologies across various applications.
Emerging Issues in NGS Technology
As the landscape of genomic research continues to evolve, various emerging issues surrounding Next-Generation Sequencing methodologies require attention. One pressing challenge is the validation of machine learning algorithms utilized in analyzing NGS data, which introduces an additional layer of complexity in ensuring accurate and reliable results. The NGS Quality Initiative is responsive to these emerging trends, striving to develop methodologies and strategies that accommodate the integration of advanced computational tools into laboratory workflows.
Furthermore, the concept of agnostic pathogen detection exemplifies how NGS is redefining the paradigm of infectious disease diagnosis. Laboratories are increasingly called upon to implement systems that can identify a broad range of pathogens without prior knowledge of the targets. This shift necessitates rigorous validation processes to ensure that the assays developed are both sensitive and specific. Through collaborative efforts and the dissemination of knowledge provided by the NGS Quality Initiative, laboratories can remain at the forefront of these developments, aligning their practices with the latest advancements in NGS technology.
The Future of NGS in Public Health
The future of Next-Generation Sequencing in public health appears promising, particularly as ongoing advancements in technology continue to empower laboratories to tackle existing challenges. With the support of the NGS Quality Initiative, labs are increasingly equipped to implement robust quality management systems that elevate the standards of genomic testing. These systems not only enhance the reliability of results but also foster a culture of excellence within public health laboratories, enabling them to respond effectively to infectious disease outbreaks and other health threats.
Moreover, the integration of NGS technologies with bioinformatics advancements signifies a transformative shift in public health surveillance and disease monitoring. As methodologies become more refined and accessible, the potential for NGS to inform real-time public health decisions grows increasingly significant. Continued collaboration and investment in workforce training, regulatory compliance, and methodological validation will shape the trajectory of NGS applications, ensuring that public health laboratories remain responsive and effective in meeting the challenges of today’s health landscape.
Ensuring Quality Control in NGS Workflows
Quality control is paramount in Next-Generation Sequencing workflows, as even minor errors can lead to significant repercussions in clinical diagnostics and public health applications. The NGS Quality Initiative emphasizes the necessity of establishing thorough quality management protocols that encompass every aspect of the sequencing process, from sample collection to data interpretation. By implementing rigorous quality checks and validations, laboratories can enhance the reliability of their results, thus building confidence among stakeholders and the public.
Moreover, the evolution of quality control measures must keep pace with advancements in sequencing technology and bioinformatics. As new sequencing platforms enter the market and analytical methods become more sophisticated, laboratories are challenged to adapt their quality assurance practices accordingly. The NGS Quality Initiative provides a framework that helps labs develop adaptable quality control strategies, ensuring that they remain compliant and effective in an ever-changing environment. This proactive approach to quality assurance positions laboratories to better navigate the intricacies of NGS and uphold high standards in their operations.
Frequently Asked Questions
What is the Next-Generation Sequencing Quality Initiative and its significance for clinical laboratories?
The Next-Generation Sequencing (NGS) Quality Initiative is an essential program developed by the CDC and the Association of Public Health Laboratories (APHL) to address laboratory challenges in implementing NGS technologies. It provides tools and resources to help laboratories create effective quality management systems, ensuring compliance with regulatory standards and enhancing the reliability of NGS results in clinical settings.
How does bioinformatics advancement impact Next-Generation Sequencing in public health laboratories?
Advancements in bioinformatics play a critical role in Next-Generation Sequencing (NGS) within public health laboratories by enhancing data analysis capabilities and streamlining the interpretation of complex genomic data. These improvements help laboratories validate NGS assays more effectively, ensuring accurate pathogen detection and monitoring in public health initiatives.
What are the challenges related to NGS method validation in clinical laboratories?
NGS method validation in clinical laboratories presents several challenges, primarily due to variability in sample types and the rigor of quality control standards. As new sequencing platforms and chemistries emerge, validating these methods becomes increasingly complex, necessitating specialized knowledge and resources to ensure high-quality outcomes.
Why is personnel retention a major challenge for Next-Generation Sequencing implementation?
Personnel retention is a significant challenge for Next-Generation Sequencing (NGS) implementation because the field requires a highly skilled workforce with specialized knowledge in genomics and bioinformatics. According to a 2021 survey by the Association of Public Health Laboratories, 30% of public health laboratory staff planned to leave the workforce within five years, highlighting the need for training and support in this evolving landscape.
What tools does the NGS Quality Initiative provide for method validation?
The NGS Quality Initiative offers several essential tools for method validation, including the QMS Assessment Tool, NGS Method Validation Plan, and NGS Method Validation Standard Operating Procedures (SOP). These resources are designed to assist laboratories regardless of their specific sequencing platform, ensuring a consistent approach to quality management and compliance.
How do emerging sequencing methodologies affect the NGS workflow in public health?
Emerging sequencing methodologies, including target and agnostic approaches, complicate the NGS workflow in public health by introducing variability in results and increasing the necessity for thorough method validation. This evolution demands that laboratories adapt their processes and maintain rigorous quality standards to ensure the reliability of NGS outcomes in public health surveillance.
What is the role of training and competency evaluation in the NGS Quality Initiative?
Training and competency evaluation are crucial components of the NGS Quality Initiative, addressing the workforce challenges in clinical and public health laboratories. The initiative provides training programs and resources aimed at enhancing the skills of laboratory personnel, ensuring they are well-equipped to utilize NGS technologies effectively and maintain high-quality results.
What are the long-term goals of the NGS Quality Initiative for public health laboratories?
The long-term goals of the NGS Quality Initiative include continuously adapting to the rapidly evolving landscape of genomics and bioinformatics, providing public health laboratories with updated tools and guidance. The initiative aims to enhance the quality and reliability of NGS applications, improve workforce training, and ensure compliance with regulatory standards in diverse laboratory settings.
| Key Point | Description |
|---|---|
| NGS Quality Initiative | A CDC and APHL initiative aimed at addressing challenges in laboratory implementation of NGS. |
| Laboratory Challenges | Includes complex regulatory frameworks, quality control issues, and sample variability affecting NGS assay validation. |
| Technological Advances | Involves new platforms, chemistries, and bioinformatics innovations that enhance NGS capabilities. |
| Workforce Management | Lack of skilled personnel remains a major barrier, with 30% of surveyed health lab staff planning to leave soon. |
| Training Tools | The initiative provides training and competency tools to help laboratories develop skilled personnel. |
| Support Documentation | Includes widely used resources like QMS Assessment Tool, NGS Method Validation Plan, and SOPs. |
| Compliance Complexity | Maintaining compliance with regulatory standards remains challenging due to evolving workflows and technologies. |
Summary
Next-Generation Sequencing (NGS) is a rapidly evolving technology that offers significant advancements in genetics and genomics. The NGS Quality Initiative is crucial in addressing the various challenges clinical and public health laboratories face when implementing these advanced sequencing techniques. By providing essential tools, resources, and training, the initiative ensures quality management systems are robust and compliant with regulatory frameworks. As NGS technology continues to develop, it is vital for labs to adapt and overcome personnel and technological challenges to maintain high-quality standards and effective public health responses.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.