The HPV vaccine is a remarkable medical innovation that has significantly decreased the incidence of cervical cancer among women, with a reduction rate of nearly 90% in those vaccinated during adolescence. This vaccine has undergone extensive research, with over 70 randomized controlled trials demonstrating its effectiveness and safety, and more than 135 million doses administered in the United States alone. Recently, the Advisory Committee on Immunization Practices (ACIP) announced plans for a comprehensive review of the HPV vaccine effectiveness, a reassessment that raises questions about its long-established benefits. Despite the robust evidence supporting the HPV vaccine’s role in cervical cancer prevention, the new focus on reevaluation may create unnecessary uncertainty around its safety, leading to possible hesitancy in vaccination. As cervical cancer remains a pressing public health issue, understanding the HPV vaccine’s efficacy and safety remains vital not only for women but for all individuals as the discussions continue.
The inoculation against human papillomavirus (HPV) plays a crucial role in combatting various cancers, notably cervical cancer. This childhood vaccine has been pivotal in public health initiatives aiming to mitigate cancer risks associated with HPV infections. Alongside its effectiveness in preventing high-grade precancerous lesions, the safety profile of the vaccine has been thoroughly assessed, with global health authorities endorsing its use. As the new working group under the ACIP begins its scrutiny of vaccine efficacy reviews, it is essential to recognize the established data that underscores the HPV vaccine’s impact on cancer prevention. The health community is urged to maintain clarity on the vaccine’s benefits to ensure it remains an integral part of childhood immunization schedules.
Understanding HPV Vaccine Effectiveness in Cervical Cancer Prevention
The effectiveness of the HPV vaccine in preventing cervical cancer has been well-established through extensive research. Studies have shown that the HPV vaccine reduces the incidence of cervical cancer by almost 90% among women who received the vaccine during their adolescent years. With over 135 million doses administered and backed by more than 70 randomized controlled trials, the evidence indicates that the vaccine is not only effective but also vital for cancer prevention. Countries with established vaccination programs have reported significant drops in cancer cases, reinforcing the vaccine’s crucial role in public health.
Specifically, studies in Sweden and Denmark have revealed that women vaccinated before the age of 17 face an 88% lower risk of developing invasive cervical cancer, while England noted an astounding 97% reduction in high-grade precancerous lesions among vaccinated girls. These findings collectively support the conclusion that the HPV vaccine is an essential tool for cervical cancer prevention, aligning with recommendations from medical authorities and public health organizations.
Safety of the HPV Vaccine: What the Data Reveals
One of the most frequently raised concerns about vaccinations, including the HPV vaccine, is safety. However, an extensive body of evidence collected from clinical trials and post-marketing surveillance demonstrates that the HPV vaccine is extremely safe. The World Health Organization (WHO) has classified the vaccine as safe, indicating that serious adverse effects are minimal and typically are limited to hypersensitivity reactions, occurring at rates of around three per million doses administered. Such findings underscore the considerable risk-benefit ratio favoring vaccination over potential adverse outcomes.
Moreover, the HPV vaccine has been subject to rigorous evaluation by safety monitoring boards, including the Global Advisory Committee on Vaccine Safety. The data from hundreds of millions of doses administered globally shows no meaningful connection between the vaccine and any long-term health issues. In fact, comparative studies involving saline placebo controls have produced indistinguishable safety results compared to those using adjuvants. This reaffirms the safety and efficacy profile of the HPV vaccine as consistently reliable.
The Advisory Committee on Immunization Practices: New Changes and Challenges
The recent changes in the Advisory Committee on Immunization Practices (ACIP) could potentially influence public perception and acceptance of the HPV vaccine. Following an overhaul in June 2025, a newly formed working group was tasked with a comprehensive review of the HPV vaccine’s efficacy and safety. This extensive reevaluation has raised concerns of bias, especially considering the working group’s inclusion of members with financial ties to vaccine litigation against the manufacturers. Such conflicts risk undermining trust in the established scientific consensus regarding HPV vaccines.
The charter established for the working group appears to question the vaccine’s value rather than refine existing policy, which could create further uncertainty about the HPV vaccine’s recommendation. Health professionals worry that this prolonged examination may lead to a downgrading of the HPV vaccine’s recommendation, potentially impacting vaccination rates and, consequently, cancer prevention outcomes. In an environment where vaccine hesitancy already poses significant public health challenges, maintaining a clear and confident recommendation is vital.
The Importance of HPV Vaccination in Boys and Men
While HPV is often perceived as a women’s health issue due to its association with cervical cancer, it is crucial to recognize that HPV also causes significant health problems in males, including anal, penile, and throat cancers. In fact, approximately 23,000 cancers attributable to HPV occur annually in men in the United States alone. Recent shifts in vaccination perspectives emphasize the importance of vaccinating boys and men against HPV to prevent these cancers.
Current discussions around single-dose vaccinations highlight the need for further data on the effectiveness of HPV vaccines in males. While the existing evidence supports the efficacy of the HPV vaccine in women, the lack of single-dose studies specifically targeting males remains an important gap. The overall impact of vaccinating boys against HPV is considerable, as it not only protects individual health but also contributes to achieving herd immunity within the community, ultimately reducing the risk for everyone.
Evidence-Based Research Supporting HPV Vaccine Adoption
Evidence from population-based studies strongly supports the efficacy of the HPV vaccine in reducing cancer incidence rates. For instance, countries like Australia and Sweden have seen dramatic declines in genital warts and precancerous cervical lesions following the implementation of their national HPV vaccination programs, demonstrating the vaccine’s contribution to public health. The data shows that as vaccination rates increase, the rates of HPV-related diseases can decrease significantly.
Furthermore, new longitudinal studies indicate that in regions where HPV vaccination coverage exceeds 90%, women can reduce their cervical cancer screening frequency, allowing for less invasive procedures and healthcare costs over time. These findings emphasize the vaccine’s long-term benefits not just for individuals but also for the healthcare system as a whole, warranting urgent action to increase vaccination uptake among eligible populations.
Debunking Myths: Misinformation Surrounding the HPV Vaccine
Despite overwhelming scientific evidence supporting the HPV vaccine, misinformation persists, fueling vaccine hesitancy. Common myths include claims of the vaccine causing serious health issues or being unnecessary due to the belief that HPV is not widespread. In reality, data indicate that HPV is a highly prevalent virus, with high-risk types responsible for the majority of cervical and other HPV-related cancers. Misinformation undermines the importance of vaccination, making it essential for healthcare providers to educate the public based on established research findings.
Health campaigns aimed at dispelling myths about the HPV vaccine must focus on factual information regarding its role in cancer prevention and safety. Engaging community leaders, educators, and medical professionals in advocacy and outreach can help frame the narrative positively around vaccination, counteracting skepticism and promoting informed choices. Achieving higher vaccination rates depends on accurately addressing misconceptions and reinforcing the collective responsibility in preventing HPV-related cancers.
The Role of HPV Vaccination in Global Cancer Control
HPV vaccination is a cornerstone in global strategies aimed at reducing cancer burden worldwide. With high-risk HPV types accounting for a significant proportion of preventable cancers in women and men, effective vaccination programs could drastically decrease the incidence of these cancers globally. The WHO highlights the critical nature of integrating HPV vaccination into national health policies, especially in regions with limited access to cancer screening and treatment.
Countries that have successfully implemented HPV vaccination campaigns—such as Australia—serve as insightful models for other nations, demonstrating that comprehensive vaccination programs can lead to the near-elimination of cervical cancer as a public health issue. The global health community must continue to collaborate on scaling these programs, ensuring equitable access to vaccines and education about their benefits, ultimately working towards a future free from HPV-related cancers.
Navigating the Landscape of HPV Vaccination Policy Changes
The evolving landscape of HPV vaccination policies brings both opportunities and challenges for healthcare providers and patients. With recent ACIP changes provoking debate over HPV vaccine recommendations, it is important for clinicians to remain informed about the evidence supporting vaccination. Clear guidelines and communication strategies are essential for effectively navigating these developments to maintain high immunization rates.
Providers must advocate for fair assessments of the HPV vaccine’s value grounded in robust scientific evidence and continue promoting the importance of vaccination to parents and adolescents. Building strong relationships with families and addressing their concerns with transparent, evidence-based information can foster trust and ensure that vaccination rates do not decline amid policy shifts, eventually safeguarding public health.
Future Directions: HPV Vaccine Research and Recommendations
Looking forward, ongoing research into HPV vaccine efficacy, especially regarding single-dose regimens, will be crucial in refining vaccination strategies. Comprehensive studies targeting both genders and diverse populations must continue to evaluate long-term protection levels and adjust recommendations accordingly. These insights will shape future vaccination schedules and policies, possibly leading to simpler, more cost-effective approaches for administering the vaccine.
The health community must also prioritize communicating research findings to the public clearly and effectively. By addressing community concerns, offering reassurance about the vaccine’s safety and effectiveness, and involving stakeholders in discussion, health authorities can better navigate public perceptions and foster an environment conducive to higher vaccination uptake. Continuous engagement and adaptability are crucial for ensuring that the HPV vaccine continues to serve its role in cancer prevention globally.
Frequently Asked Questions
What is the effectiveness of the HPV vaccine in preventing cervical cancer?
The HPV vaccine has been shown to reduce the incidence of cervical cancer by nearly 90% in women vaccinated as adolescents. Studies, including population-based research, confirm that this vaccine prevents cancer on a large scale, with significant reductions in cervical cancer cases in countries with established vaccination programs.
How safe is the HPV vaccine based on recent reviews?
The HPV vaccine is classified as ‘extremely safe’ by the World Health Organization’s Global Advisory Committee on Vaccine Safety. Safety data from over 135 million doses administered in the U.S. indicate no serious adverse reactions beyond the rare possibility of severe allergies. Ongoing reviews continue to support the vaccine’s safety profile.
What recommendations has the Advisory Committee on Immunization Practices made regarding the HPV vaccine?
The Advisory Committee on Immunization Practices (ACIP) has historically endorsed the HPV vaccine as vital for cervical cancer prevention. However, a new working group under ACIP is conducting a comprehensive review of the vaccine’s effectiveness and safety, including previously established data on its efficacy and safety.
Is there ongoing research into the HPV vaccine’s efficacy, particularly regarding single-dose schedules?
Yes, ongoing research is focusing on whether a single dose of the HPV vaccine can provide long-term protection comparable to the traditional two or three-dose schedules. Preliminary evidence shows that a single dose may be nearly as effective, but comprehensive data is still being evaluated.
What does the evidence show about the HPV vaccine’s effectiveness across different populations?
Evidence from multiple studies indicates that the HPV vaccine is effective across diverse populations. For instance, studies show an 88% reduction in invasive cervical cancer among vaccinated women in Sweden and significant declines in precancerous lesions in England and Denmark, demonstrating its global effectiveness.
What are the potential risks associated with the HPV vaccine that are being reviewed by ACIP?
The ACIP’s new working group is set to review concerns about potential toxicity from the HPV vaccine, including adjuvant safety and possible contaminants. However, past studies indicate that the aluminum adjuvants used in HPV vaccines are safe and lower in quantity than normal exposure from everyday sources.
How does the HPV vaccine impact cancer prevention for men?
While the HPV vaccine is crucial for preventing cervical cancer in women, it also plays a vital role in preventing HPV-related cancers in men, such as throat and penile cancers. Advocating for male vaccination is important, though recent assessments have highlighted gaps in data regarding single-dose efficacy for males.
What demographic trends exist regarding HPV vaccine uptake in the United States?
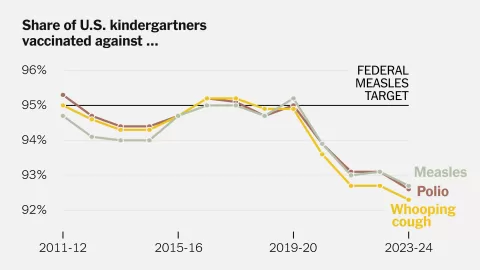
Current trends show that only 62.9% of U.S. adolescents complete the HPV vaccine series, which is lower than coverage for other vaccines at the same age. Efforts to improve HPV vaccination rates are critical to preventing future cancer cases.
| Key Points |
|---|
| The HPV vaccine significantly reduces cervical cancer rates in vaccinated women by nearly 90%. |
| Over 135 million HPV vaccine doses have been administered in the US, backed by extensive research from randomized controlled trials. |
| A new working group under ACIP is set to review the HPV vaccine’s efficacy, safety, and dosing schedules, including single dose efficacy. |
| Real-world studies show profound reductions in cervical cancer rates linked to vaccination, with countries achieving up to a 93% drop in cases. |
| The HPV vaccine is endorsed as extremely safe by the WHO, with serious adverse reactions being exceedingly rare. |
| Concerns about the working group’s focus on vaccine toxicity and re-evaluating benefits could create confusion and decrease vaccination rates. |
| The re-evaluation process might prioritize biases over scientific evidence, potentially influencing public health negatively. |
Summary
The HPV vaccine is a crucial tool in the fight against cancer, proven to significantly reduce the incidence of cervical cancer among vaccinated girls. The recent decision by a newly formed working group to comprehensively review the HPV vaccine’s efficacy and safety highlights the importance of continuous evaluation in public health. However, the extensive data supporting the vaccine’s safety and effectiveness raises concerns about the motivations behind this re-evaluation, as it could undermine public confidence and decrease vaccination rates. Continuous public awareness and education about the HPV vaccine are essential to maintain its role in cancer prevention and to ensure that future generations can benefit from its life-saving effects.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.