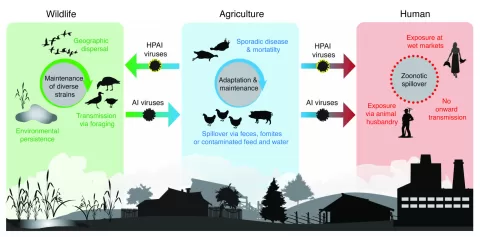
Highly Pathogenic Avian Influenza H5N1 has emerged as a critical concern in the realm of infectious diseases, particularly after its recent spread in Canadian wildlife. In 2024, British Columbia faced a significant outbreak involving HPAI H5N1, specifically the clade 2.3.4.4b, which is notorious for its high pathogenicity in both wild birds and poultry. This strain, notably the genotype D1.1, arose from a complex reassortment of viruses, reflecting a concerning adaptation that has facilitated its rapid dissemination. Tracking the impacts and evolution of HPAI H5N1 in wild birds is crucial, especially given the potential for human infection, as seen with reported cases in British Columbia. Understanding these dynamics is essential for public health and wildlife management strategies to mitigate the risks of avian influenza transmission.
The emergence of avian flu, specifically the strain known as HPAI H5N1, presents a significant threat to both avian and human populations alike. Often referred to as bird flu, this virus has gained attention due to its high mortality rates, particularly in poultry, and its sporadic infections in humans. In recent times, the presence of H5N1 in wild bird populations has led to increased surveillance efforts aimed at controlling outbreaks. Particularly alarming is the specific clade 2.3.4.4b, documented in British Columbia. This avian influenza strain has raised flags among health officials due to its potential for mutation and spillover into human hosts, making it a priority for further research and monitoring.
Understanding Highly Pathogenic Avian Influenza H5N1 in Wild Birds
Highly pathogenic avian influenza A(H5N1) has emerged as a significant threat to avian populations worldwide. It is crucial to understand its spread, especially among wild birds, as these species often serve as reservoirs and vectors for the virus. The recent emergence of the H5N1 clade 2.3.4.4b, particularly genotype D1.1, in British Columbia in 2024, underscores the importance of avian monitoring programs. These programs help track the virus’s spread and mutations in avian populations, vital for protecting both wildlife and poultry industries.
The introduction of HPAI H5N1 in British Columbia can be traced back to its entry through migratory pathways such as the East Atlantic and Pacific Flyways. The ability of wild birds to carry and disseminate the virus can complicate management efforts, as these birds frequently travel long distances. This complexity is compounded by the fact that H5N1 is not only affecting wild populations but has also led to spillover events into domestic poultry and potential human cases, emphasizing the interconnectedness of avian health and public health.
The Impact of H5N1 Clade 2.3.4.4b on Poultry and Humans
The presence of H5N1 clade 2.3.4.4b in wild birds has serious implications for poultry health and agriculture. In British Columbia, the detection of this strain has resulted in numerous outbreaks among poultry farms, causing significant economic losses and food safety concerns. The reassortment seen in genotype D1.1 indicates that the virus is evolving and may adapt further to domestic environments. As a result, stringent biosecurity measures are recommended for poultry farmers to minimize the risk of infection from wild bird populations.
Moreover, the transmission of HPAI H5N1 from birds to humans raises critical public health concerns. The confirmed case of a teenager in Fraser Valley highlights the need for vigilance and preparedness against potential outbreaks among humans. While cases of avian influenza in humans remain rare, the adaptability of certain strains, like D1.1, could lead to increased transmissibility and severity if the virus continues to evolve. Public health agencies must remain proactive in monitoring human cases and providing timely information to reduce the risk of infection.
Monitoring and Surveillance of HPAI in British Columbia
The British Columbia Wildlife Avian Influenza Surveillance Program plays a crucial role in monitoring the spread of highly pathogenic avian influenza. Through the application of quantitative reverse transcription PCR and whole-genome sequencing, the program can identify and analyze viral strains circulating among wild birds. Monitoring efforts during the fall of 2024 revealed 57 confirmed detections of HPAI H5N1, mostly in populations like cackling and Canada geese, which are common in the area. These findings emphasize the necessity for ongoing surveillance to detect new outbreaks promptly.
By collaborating with local wildlife organizations, researchers can enhance detection methods for HPAI H5N1 in wild birds. This research aids in understanding the dynamics of the virus, including outbreaks linked to specific seasons or geographic locations. Continuous surveillance allows for a better understanding of the relationship between wild birds and farmed poultry, thereby informing strategic responses to potential outbreaks and protecting both wildlife and agricultural sectors.
The Evolution of HPAI Genotypes in North America
The evolution of HPAI genotypes in North America, especially clade 2.3.4.4b, illustrates the complexities of avian influenza dynamics. In British Columbia, the emergence of genotype D1.1 signifies a major shift in the virus’s genetic landscape. This genotype’s reassortment from earlier introduced A3 lineage viruses suggests that the virus adapts continuously, potentially leading to novel characteristics such as enhanced transmissibility or virulence.
Understanding the lineage and evolution of H5N1 is critical for effective management policies. The interactions between various strains from different geographical areas, as seen with H5N1’s reassortment events, require constant examination. Enhanced genetic studies could provide insights into how viral evolution affects both wild bird populations and the broader ecosystem, potentially influencing strategies for controlling avian influenza across borders.
Avian Influenza: A Global Perspective
Avian influenza is not just a regional issue; it is a global health concern that demands an international approach to management and prevention. Outbreaks of HPAI, particularly in migratory birds, can quickly lead to widespread epidemics affecting domestic poultry and, in some cases, humans. The interconnectedness of ecosystems and human activity further complicates efforts to control the spread of H5N1 and similar strains.
International surveillance and collaboration among countries are crucial for understanding and mitigating the impacts of avian influenza. Sharing data and resources, along with developing standardized protocols for monitoring and response, can enhance the global effort to address HPAI. By fostering a comprehensive approach that integrates veterinary, wildlife, and public health perspectives, stakeholders can better prepare for and respond to future outbreaks.
The Role of Public Health in Avian Influenza Prevention
Public health plays a critical role in preventing and managing outbreaks of avian influenza in both domestic and human populations. Surveillance programs not only help track the spread of HPAI H5N1 in avian species but also monitor potential spillover events that could lead to human infections. Education and awareness campaigns can inform communities about the signs of avian influenza in birds and the necessary precautions to take when interacting with wildlife.
In conjunction with wildlife management practices, public health authorities must develop clear response strategies in the event of a confirmed case in humans or significant outbreaks in poultry. These strategies may include vaccination programs, public advisories, and rapid response teams to contain and address any emergent health threats. By reinforcing the connection between public health and wildlife management, communities can better safeguard against the risks posed by HPAI.
Understanding Genotype D1.1 and Its Implications
The identification of genotype D1.1 as a distinct variant of HPAI H5N1 highlights the importance of continuous genetic analysis in understanding the virus’s behavior. This genotype emerged as the predominant strain linked to recent outbreaks in British Columbia, raising concerns about its potential impact on both avian and human populations. Genetic features may confer advantages that enhance transmissibility among birds and increase the risk of spillover to humans.
Further studies into genotype D1.1 are critical to assessing its implications for host range and infectivity. Researchers must investigate how this lineage interacts with both wild and domestic species, as well as its potential to adapt in human populations. Understanding these dynamics will be pivotal for developing effective vaccines and intervention strategies to mitigate the risks posed by this evolving virus.
Biosecurity Measures for Poultry Farmers
In light of the HPAI H5N1 outbreaks, implementing stringent biosecurity measures is essential for poultry farmers. These measures should include monitoring and controlling the movement of birds, maintaining cleanliness within enclosures, and educating farm workers about the signs of avian influenza. By adopting these practices, farmers can limit exposure to HPAI and prevent outbreaks from spreading to their flocks.
Additionally, collaboration with veterinarians and wildlife health professionals can enhance biosecurity protocols, ensuring they are tailored to regional conditions and risks. Farmers should also stay informed about the latest developments regarding avian influenza, including updates on circulating genotypes such as D1.1, to adjust their biosecurity measures accordingly. A proactive approach will help protect agricultural interests and public health in the face of this ongoing threat.
Future Directions in Avian Influenza Research
Research into avian influenza continues to be a priority for scientists and public health officials, especially in the context of emerging strains like HPAI H5N1 clade 2.3.4.4b. Future studies must focus on tracking the migration patterns of wild bird populations and understanding their role in the epidemiology of avian influenza. This research will aid in predicting potential outbreaks and implementing preventative measures effectively.
Moreover, advancements in genomic technologies can provide deeper insights into virus evolution and pathogenicity. By investigating the molecular mechanisms that allow H5N1 to infect different species, researchers can contribute to developing vaccines and therapeutics. Future efforts should also include a holistic approach that integrates ecosystem health with public health strategies, acknowledging that avian influenza is not merely a veterinary issue but a multifaceted global health challenge.
Frequently Asked Questions
What is Highly Pathogenic Avian Influenza H5N1 and its impact on wild birds in British Columbia?
Highly Pathogenic Avian Influenza H5N1, specifically clade 2.3.4.4b, significantly impacted wild birds in British Columbia during 2024. This virus was first introduced via the East Atlantic Flyway and caused multiple waves of infection, predominantly affecting species like cackling and Canada geese. The emergence of new genotypes such as D1.1 highlights the ongoing risk of HPAI H5N1 in wildlife populations.
How does HPAI H5N1 clade 2.3.4.4b affect poultry in British Columbia after its introduction?
The introduction of HPAI H5N1 clade 2.3.4.4b to British Columbia resulted in numerous spillover events into domestic poultry, leading to significant outbreaks. Different genotypes were detected, with genotype D1.1 showing a notable association with poultry infections alongside wild birds during 2024.
What are the human health risks associated with H5N1 genotype D1.1 in British Columbia?
The detection of H5N1 genotype D1.1 in a human case in British Columbia in November 2024 raises concerns about avian influenza human infections. This incident underscores the potential for transmission from infected wild birds and poultry to humans, prompting increased surveillance and research into the dynamics of HPAI H5N1 transmission.
What is the significance of HPAI H5N1 surveillance in wild birds regarding public health?
Surveillance of HPAI H5N1 in wild birds is crucial for understanding the virus’s ecology and potential human health impacts. The British Columbia Wildlife Avian Influenza Surveillance Program monitors viral activity and genotypes, such as D1.1, to inform public health responses and prevent possible spillover events into human populations.
How did genotype D1.1 of HPAI H5N1 emerge in British Columbia?
Genotype D1.1 of HPAI H5N1 emerged as a result of reassortment between Eurasian A3 lineage viruses and North American viruses. Its surge in British Columbia during fall 2024 shows the dynamic nature of avian influenza viruses, highlighting the need to track such genetic changes for effective management and control.
What measures are being taken to monitor HPAI H5N1 in British Columbia?
Monitoring HPAI H5N1 in British Columbia involves systematic surveillance of wild birds, including testing for the virus through PCR and whole-genome sequencing. Initiatives driven by local health authorities aim to quickly identify outbreaks and assess the risks to both wildlife and poultry populations.
Why is H5N1 clade 2.3.4.4b particularly concerning for wildlife and agriculture?
H5N1 clade 2.3.4.4b is particularly concerning due to its high pathogenicity in birds and its potential to cause severe outbreaks in poultry, significantly disrupting agricultural practices. The ongoing mutations and reassortment, evidenced by cases like genotype D1.1, pose further challenges for wildlife and agricultural health.
What is the connection between HPAI H5N1 in wild birds and potential zoonotic transmission?
The connection between HPAI H5N1 in wild birds and zoonotic transmission is critical, as evidenced by human cases linked to the virus. Understanding this connection is essential for public health efforts focused on preventing human infections from spillover events originating from infected birds or poultry.
| Key Points |
|---|
| The H5N1 A clade 2.3.4.4b was introduced to North America in 2022 and has been circulating among wild birds in British Columbia. |
| As of the end of 2024, British Columbia experienced 4 waves of H5N1 infections, leading to multiple spillover events into poultry. |
| The predominant genotype in 2024 was D1.1, which emerged from reassortment between Eurasian and North American viruses. |
| A single case of H5N1 infection in a human was confirmed in November 2024, linked to detections in wild birds. |
| Further research is necessary to understand the implications of genotype D1.1 on the transmission dynamics of H5N1. |
Summary
Highly Pathogenic Avian Influenza H5N1 remains a significant concern, as evidenced by its emergence in wild birds and its recent spread to humans in British Columbia. The occurrence of the D1.1 genotype highlights the virus’s adaptability and the potential risks it poses to both poultry and human health. Continued surveillance and research are crucial to managing and understanding the evolving dynamics of H5N1, especially regarding its transmission pathways. As new strains develop, the public health response will need to adapt accordingly to mitigate the risks associated with this highly pathogenic virus.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.