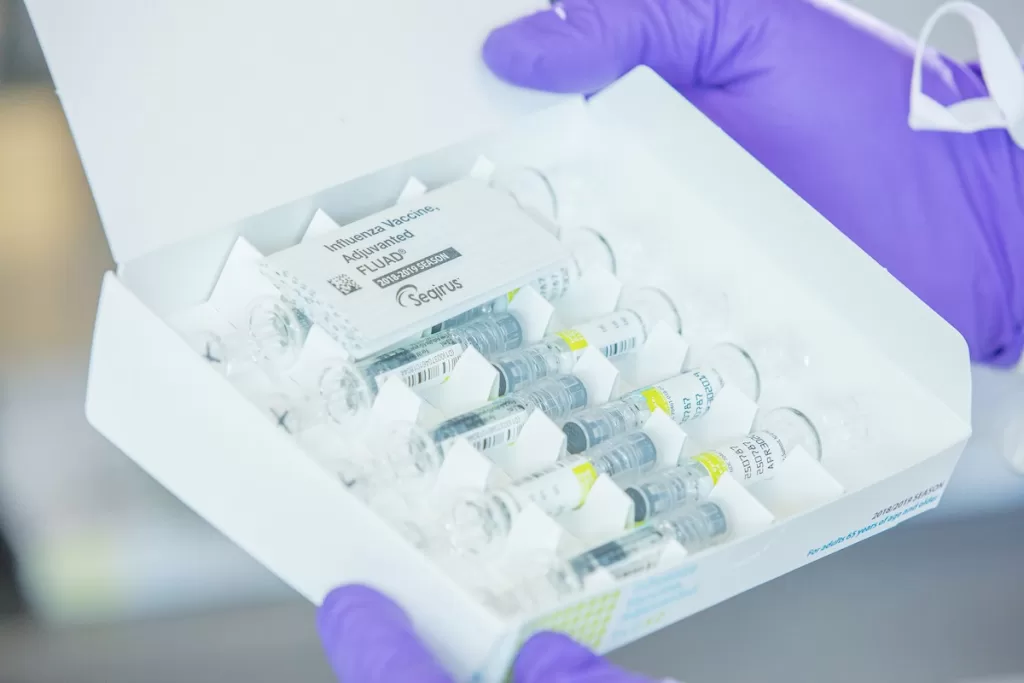
Flu vaccine shipments are underway as companies gear up for the 2025-26 flu season, marking a crucial step in preparing for influenza outbreaks. With three major flu vaccine companies — GSK, CSL Seqirus, and Sanofi — declaring the commencement of their shipments, healthcare providers and pharmacies are eager to stock these essential vaccines. Following a recent CDC flu update that highlighted the severity of the past season, the importance of timely vaccination has never been more evident. Last season witnessed the alarming reality of pediatric flu deaths reaching unprecedented levels, underscoring the need for proactive measures. As we anticipate the upcoming season, staying informed about influenza vaccine news and developments will be key for families and healthcare professionals alike.
As the 2025-26 influenza season approaches, the distribution of flu vaccines is a focal point in public health discussions. Vaccine manufacturers are starting to dispatch their products, ensuring that communities have access to necessary immunizations against the flu virus. The recent updates from the CDC on flu transmission and the implications of last year’s severe flu season emphasize the urgency for vaccinations. In light of significant pediatric flu deaths reported last season, it becomes crucial for both caregivers and healthcare practitioners to be aware of available options for flu prevention. These early shipments not only signify a proactive approach to public health but also highlight the ongoing efforts to protect vulnerable populations from the influenza virus.
Flu Vaccine Shipments: Preparing for a New Season
As summer begins to wane, the surge in flu vaccine shipments signals the imminent arrival of the 2025-26 flu season. Major flu vaccine companies such as GSK, CSL Seqirus, and Sanofi have commenced the distribution of their vaccine doses to healthcare facilities across the United States. This proactive approach is crucial as it allows healthcare providers ample time to prepare for vaccination campaigns, especially after an unprecedentedly severe flu season last year. The Centers for Disease Control and Prevention (CDC) has noted a historically high rate of hospitalizations, further underscoring the importance of timely vaccine availability.
The roll-out of these flu vaccines is not just a logistics challenge; it reflects a comprehensive public health strategy aimed at reducing flu-related morbidity and mortality. The CDC has urged healthcare providers to advertise the availability of these vaccines to ensure that communities are informed and prepared for immunization efforts. With pediatric flu deaths rising to alarming levels last year, swift action in distribution is essential for safeguarding vulnerable populations, notably children.
CDC Flu Updates: Insights from Recent Seasons
The CDC has been at the forefront of monitoring influenza trends, and their updates play an invaluable role in shaping public health responses. Each season provides critical insights, especially after the high-severity flu season of 2024-25. The agency’s recent reports document not only the uptick in hospitalizations but also highlight the disturbing number of pediatric flu deaths. As the 2025-26 flu season approaches, the CDC has recommended that vaccinations begin as soon as vaccines are available, particularly in light of past pediatric outcomes where many affected had no known underlying conditions.
In continual efforts to combat influenza, the CDC also updates its vaccine guidance regularly, which includes information on the latest vaccine formulations and recommendations for specific populations. By actively disseminating this information, the CDC aims to empower healthcare providers and patients alike to make informed decisions regarding flu vaccinations, ultimately aiming to mitigate the health impacts of the flu.
Changes in Flu Vaccine Offerings for 2025-26
With advancements in vaccination technology, significant modifications have been introduced for the 2025-26 flu season. Among these changes is the increased accessibility of FluMist, a needle-free option, which is now available for self-administration or by caregivers, expanding the horizon for those hesitant about traditional vaccinations. The FDA’s approval of this method has been a game changer in enhancing vaccine uptake, especially among individuals who may fear needles.
Moreover, the CDC has aligned its recommendations with this new availability, suggesting that this option could significantly increase vaccination rates among children and adults alike. The adaptation of Flublok’s age recommendation also marks a pivotal moment, allowing use in children as young as nine years old. These innovations aim to address public health needs, ensuring that various demographics receive adequate protection against influenza as the upcoming season unfolds.
Pediatric Flu Deaths: A Rising Concern
The alarming rise in pediatric flu deaths during the last flu season has raised significant concerns among healthcare professionals and parents alike. The CDC’s report revealed that 2024-25 saw the highest number of pediatric flu fatalities in a non-pandemic year since mandatory reporting began. This distressing trend highlights the urgent need for enhanced flu prevention strategies, particularly for children, who are among the most vulnerable to the virus.
Healthcare providers are now more than ever emphasizing the importance of vaccinations, aiming to mitigate risks for all children, especially those without prior health issues. The CDC’s focus on educating parents about the signs and symptoms of influenza, as well as promoting vaccination, is a critical component in preventing further tragedies in the younger population.
Flu Vaccine Companies: Key Players in Public Health
The 2025-26 flu season will see the contributions of major flu vaccine companies such as GSK, CSL Seqirus, and Sanofi, who play an essential role in public health efforts to combat influenza. These companies not only produce a variety of vaccines tailored for different age groups and health needs but are also responsible for ensuring that these vaccines are safely and effectively distributed across the country. Their proactive shipping strategies are particularly necessary in light of past seasons where flu severity reached alarming levels.
In addition to the logistical aspects, these companies are involved in ongoing research and development to improve vaccine efficacy year after year. Collaborations with agencies like the CDC and healthcare providers help them streamline their processes and ensure they can quickly respond to seasonal changes in flu strains, ultimately benefitting public health outcomes.
Influenza Vaccine News: Keeping the Public Informed
Influenza vaccine news is a critical focus for both healthcare providers and the general public, as timely updates can significantly influence vaccination rates and public health practices. With continuous innovations in vaccine technology and distribution protocols, staying informed is vital during flu seasons. News about upcoming vaccine shipments allows for preparedness in healthcare settings, ensuring vaccines are available ahead of peak flu activity periods.
Moreover, as the CDC releases updates, public health campaigns also aim to demystify the vaccination process and address common misconceptions among the public. Engaging communities through informative outreach can enhance understanding and compliance regarding seasonal flu shots and their benefits. This dialogue is important to ensure high vaccination coverage, which not only protects individuals but also contributes to the overall health of the community.
Community Vaccination Programs: Bridging Gaps in Immunization
Community vaccination programs are crucial in addressing barriers to immunization, especially as new flu vaccines are distributed for the 2025-26 season. These programs often partner with local healthcare providers to offer accessible and affordable vaccination options, particularly in underserved areas. As flu vaccine shipments roll out, these initiatives aim to ensure that all segments of the population can access flu shots, promoting public health and reducing the incidence of flu-related complications.
Moreover, community programs often engage with schools, churches, and local organizations to raise awareness and increase participation in vaccination efforts. By providing education on the importance of flu vaccination, these programs not only enhance uptake but also combat misinformation that may deter community members from fully participating in immunization campaigns.
The Importance of Timely Vaccination Against Influenza
Timely vaccination against influenza is highlighted as a cornerstone of public health strategy for the upcoming 2025-26 season. With the recent CDC flu update emphasizing the urgent need for flu shots due to high pediatric mortality rates from last season, the importance of early vaccinations cannot be overstated. Vaccinating early in the season allows the immune system to build up defenses against the virus before peak flu activity occurs.
Healthcare experts advocate that not only should individuals schedule their vaccinations during the early fall months, but they should also encourage their friends and family to do the same. By fostering a culture of vaccination, communities can diminish the potential strain on healthcare systems during flu season, ultimately allowing for a more focused response to public health concerns.
Preparing for Severe Flu Seasons: Lessons Learned
The experience from the 2024-25 flu season serves as a critical learning opportunity for public health officials and organizations. The unprecedented severity of last year’s flu outbreak highlighted vulnerabilities within healthcare systems and the need for improved preparedness strategies as we approach the 2025-26 season. Lessons learned include the importance of rapid vaccine development and clear communication regarding vaccine availability and effectiveness to healthcare providers.
Moreover, the rise in pediatric flu deaths has catalyzed a stronger emphasis on prevention strategies for vulnerable populations. Enhanced communication among vaccine companies, public health bodies, and communities is vital to ensure that the challenges faced in the last flu season are not repeated. By applying these lessons, we can strive to reduce flu-related mortality rates and increase the overall effectiveness of flu vaccination campaigns.
Frequently Asked Questions
What can we expect from flu vaccine shipments for the 2025-26 flu season?
Flu vaccine shipments for the 2025-26 flu season have begun, with major companies like GSK, CSL Seqirus, and Sanofi now distributing their vaccines to healthcare providers and pharmacies. This timely delivery is critical given the high severity of last year’s flu season.
How are flu vaccine shipments addressing the high pediatric flu deaths reported in recent seasons?
Flu vaccine shipments are essential in combating high pediatric flu deaths. The recent CDC data noted 260 pediatric fatalities in the last season, highlighting the urgency of vaccination efforts as shipments commence for the 2025-26 flu season to protect vulnerable groups.
Which companies are responsible for flu vaccine shipments for the upcoming flu season?
Three major companies—GSK, CSL Seqirus, and Sanofi—have begun their flu vaccine shipments for the 2025-26 season. Each company offers various vaccine options tailored for different age groups, ensuring broader vaccination coverage.
What vaccines are included in the current flu vaccine shipments from GSK?
GSK’s flu vaccine shipments for the 2025-26 season include Flulaval and Fluarix, both available in 0.5-milliliter single-dose prefilled syringes for individuals aged 6 months and older.
How does the CDC influence flu vaccine shipments?
The CDC provides updates and recommendations that influence flu vaccine shipments. Their weekly updates guide healthcare practices on vaccination strategies, especially following a season marked by severe flu cases and pediatric deaths.
What new options are available in this year’s flu vaccine shipments for self-administration?
This year’s flu vaccine shipments include a new option for self-administration: FluMist, which has been approved for individuals aged 2 to 17 to allow more accessible vaccinations through pharmacies.
What challenges are flu vaccine companies facing for the 2025-26 flu season?
Flu vaccine companies face the challenge of ensuring timely shipments and adequate supply to prepare for a potentially severe flu season, following the heightened hospitalizations and pediatric flu deaths reported last year.
Are there any significant changes in flu vaccine formulations for the upcoming season?
Yes, changes in flu vaccine formulations include an expanded age recommendation for Flublok, now approved for use in children as young as 9 years old, and the enhanced availability of FluMist for self or caregiver administration.
Why are timely flu vaccine shipments critical this year?
Timely flu vaccine shipments are crucial this year due to last season’s record hospitalization rates and pediatric fatalities, reflecting an immediate need for vaccinations ahead of the 2025-26 flu season.
How does vaccine shipment timing impact vaccinations for the 2025-26 flu season?
The timing of flu vaccine shipments directly affects the ability of healthcare providers to initiate vaccinations early in the season, which is vital for preventing widespread influenza outbreaks.
| Key Point | Details |
|---|---|
| Flu Vaccine Shipments Begin | Three companies commence flu vaccine shipments for the upcoming 2025-26 season. |
| GSK’s Offerings | GSK is shipping its trivalent vaccine (Flulaval and Fluarix) in single-dose prefilled syringes for ages 6 months and older. |
| CSL Seqirus’ Products | CSL Seqirus is shipping Flucelvax, Fluad, and Afluria vaccines, suitable for different age groups. |
| Sanofi’s Distribution | Sanofi ships vaccines to various healthcare facilities; emphasizes importance of vaccination after last year’s severe flu season. |
| Changes in Offerings | New approvals include FluMist for self-administration and expanded age for Flublok. |
Summary
Flu vaccine shipments are underway as companies prepare for the upcoming 2025-26 season. With three major vaccine producers beginning their distributions, healthcare providers are gearing up for what could be another challenging flu season, especially in the wake of last year’s high severity levels. The timely commencement of these shipments is crucial for effective vaccination campaigns, addressing the increased severity of flu seasons and preventing further health crises.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.