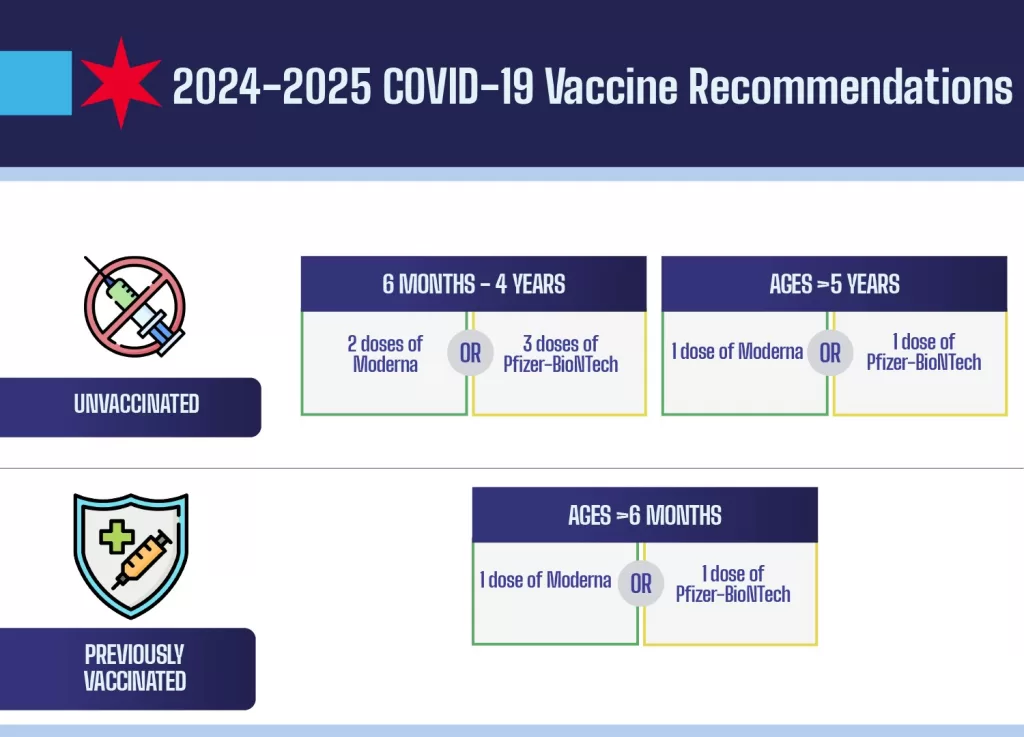
COVID vaccine availability is currently a hot topic, especially following a significant shake-up at the CDC that has raised more questions than answers. As political changes unfold, especially with the recent FDA COVID vaccine approval announcements, the public is eager for clear information on how and when they can access these vaccines. Recent updates from trusted bodies highlight the challenges facing lower-risk adults and children in obtaining their vaccinations, especially in states with stringent regulations. Furthermore, the involvement of prominent figures such as Robert F. Kennedy Jr., combined with ongoing debates surrounding public health policies, adds another layer of complexity to the already confusing landscape. As the situation develops, keeping track of COVID vaccination updates has never been more essential for individuals and families alike.
The availability of COVID immunizations has become increasingly crucial in the context of changing health governance and policies. With recent shifts in leadership at the CDC and decisions made by the FDA regarding vaccine endorsements, many find themselves searching for adequate resources to understand vaccination logistics. As we navigate these tumultuous health policy changes, it is vital to recognize the role that community pharmacies and healthcare providers play in making these vaccines accessible. Additionally, the discourse fostered by notable health advocates can influence public perception and decision-making, thereby shaping the overall vaccination strategy. Thus, staying informed about the latest vaccination schedules, eligibility criteria, and regional restrictions is essential for maximizing community health efforts.
Recent Changes in CDC Leadership
The recent upheaval at the Centers for Disease Control and Prevention (CDC) has sent ripples throughout the public health community. As Jim O’Neill was dismissed from his position as the CDC director, the appointment of his deputy to take over has raised eyebrows and questions regarding future health policies. Under the direction of Acting CDC Director O’Neill, expectations are high for how the agency will navigate the tumultuous waters left in the wake of scandal and criticism. This shake-up has significant implications for the management of the COVID pandemic, as leadership stability has been a critical factor in public trust and engagement with vaccination efforts.
Leadership transitions at the CDC are rarely straightforward, and this instance is no exception. The appointment of O’Neill follows several troubling resignations among top scientists, signaling potential upheaval in established health practices and decisions. These changes appear to align with broader political shifts and ongoing discussions within the agency regarding COVID vaccination updates. As public health guidelines evolve, the implications for upcoming health policy changes could influence everything from funding allocation to vaccination strategy implementation across the nation.
Navigating the Chaos of COVID Vaccine Availability
The current environment surrounding COVID vaccine availability is marked by confusion, particularly due to the leadership changes at the CDC. As new vaccines are approved, the pathway for accessibility remains fraught with challenges. Many individuals—especially those in lower-risk categories—are left uncertain about when and where they can receive these vaccines. It’s important to remain informed about local health policies and guidelines that dictate availability as the FDA has authorized updates that can redefine vaccination strategies.
Moreover, delays reported by major pharmacy chains like CVS complicate access to vaccines for many. With the CDC Advisory Committee on Immunization Practices (ACIP) reviews affecting which groups can receive the vaccine in specific states, individuals must stay abreast of updates. This scenario speaks to the necessity of clear communication regarding COVID vaccine availability, especially as public health agencies grapple with maintaining trust amidst ongoing policy changes and scrutiny of health directives from leaders like Robert F. Kennedy Jr.
The Implications of CDC Shake-up on Vaccine Policy
The recent shake-up at the CDC has significant implications for current vaccine policies, especially regarding COVID-19. With Robert F. Kennedy Jr. at the helm of HHS, new policy directions could further embrace controversial perspectives on vaccination safety, leading to divisions within public health narratives. As proposed studies investigating discredited claims regarding the vaccine-autism link resurface, there lies a potential for public fear to overshadow scientific consensus and established guidelines from credible health organizations like the CDC.
As health policy changes unfold, the ability of the CDC to provide accurate, unbiased vaccine information becomes crucial. The recent resignations from key roles within the agency suggest internal turmoil may affect its outreach and educational efforts. With ongoing debates surrounding vaccine efficacy and safety, it is imperative that the CDC reinforces its commitment to transparency and scientific integrity to reassure the public amidst fears propagated by emerging narratives.
FDA COVID Vaccine Approval: What You Need to Know
The landscape of COVID vaccination is evolving, especially with the FDA’s recent approvals of updated vaccines. Understanding the nuances of these approvals is essential for ensuring comprehensive vaccination programs that meet the needs of the population. The FDA’s decisions also dictate the eligibility and availability of vaccines across various states, further exacerbating disparities in access depending on regional health policies.
Knowledge of the FDA’s approval process and how it interacts with state regulations and pharmacy capabilities—like those evidenced by CVS’s delays—is critical. As pharmacies navigate the complex requirements set forth by the FDA and CDC, communities must remain vigilant, advocating for timely access to vaccinations. Future developments in vaccine availability will rely heavily on the effectiveness of these federal and state agencies to coordinate a cohesive vaccination strategy.
Health Policy Changes Affecting COVID Vaccines
As the discourse around vaccine safety and effectiveness intensifies, recent health policy changes present both opportunities and risks for the administration of COVID vaccines. Initiatives led by influential public health figures may reshape the landscape of how vaccines are perceived and distributed, emphasizing particular narratives that could either empower or undermine public health goals. For example, the potential integration of pseudoscientific claims into mainstream policy could have adverse effects on vaccination rates.
Public health policies must adapt to these evolving narratives while ensuring that scientific evidence remains at the forefront of health decisions. As the CDC navigates misinformation and seeks to uphold its credibility, the focus must rest on delivering accurate information about COVID vaccines and their importance. Engaging communities through effective communication strategies will be paramount to counteract fear and misinformation as health policies transition in response to new leadership.
Future of Vaccination amid Political Changes
The political landscape surrounding public health management in the United States has become increasingly polarized, particularly regarding vaccination initiatives. The recent changes in the CDC’s leadership may signal a shift towards more politicized health policy decisions, which could impact the accessibility and public perception of COVID vaccines. The relationship between political figures and scientific advisory committees could complicate the straightforward delivery of vaccination programs, as controversy over the credibility of certain researchers and their findings comes into play.
As policymakers navigate these turbulent waters, the future of vaccination programs will likely hinge on public trust and understanding. Ensuring clear, non-biased communication regarding vaccine safety and efficacy will be crucial in rallying both public support and compliance. Furthermore, addressing the concerns of parents and communities in relation to the updated COVID vaccines will be a priority as the nation seeks to recover from the pandemic’s far-reaching impacts.
Addressing Public Concerns about Vaccine Safety
Public concern regarding vaccine safety has been simmering, and recent changes at the CDC may intensify these fears. As misleading information about vaccines circulates, health agencies must work diligently to combat these narratives through education and outreach. Addressing the stigma surrounding vaccinations will be essential, especially with ongoing studies about the purported vaccine-autism link that have arisen from the political reshuffle.
To alleviate fears, it’s crucial that public health communications are rooted in transparency and present verifiable data that refutes common misconceptions about vaccine safety. Engaging community leaders and healthcare providers in these discussions can help build authority and trust in messaging, thus encouraging higher vaccination rates. The resurgence of scientific discussions surrounding vaccine safety represents an opportunity for both public health officials and the community to reinforce the importance of immunization.
Importance of Access to COVID Vaccines
Access to COVID vaccines remains a pressing issue as health authorities aim to reach vulnerable populations that may currently face barriers. Recent reports track delays in vaccine distribution at chains like CVS, emphasizing the importance of ensuring that eligibility and availability do not create further inequities in healthcare access. As health policies adapt to leadership changes, it’s crucial that logistical challenges in delivering vaccines be addressed head-on to prevent disparities.
Ensuring that the processes for vaccine availability consider the unique hurdles faced by underserved populations is essential in this current climate. These challenges extend beyond just obtaining vaccines; they also encompass education, transportation, and trust in the healthcare system. Public health initiatives should prioritize creating accessible channels for information and logistics around vaccinations to improve overall health outcomes within communities.
Engagement with Community Health Initiatives
engagement with community health initiatives will play a crucial role in promoting COVID vaccination efforts as we navigate the complexities of public health responses. Collaborations between local health departments, community organizations, and health professionals can help to foster trust and transparency regarding vaccine initiatives, reinforcing the commitment to public health. By tapping into local knowledge and leaders, agencies can address specific needs and concerns related to vaccine uptake.
Moreover, community-based strategies to educate and dispel myths about vaccine safety will be vital as new narratives emerge. Open dialogue forums and proactive outreach programs can assist in quelling fears and building a sense of shared responsibility around public health challenges. The future of COVID vaccination relies on not only scientific integrity but also on collective engagement and collaborative efforts to ensure that all community members receive the information and support necessary for informed health decisions.
Frequently Asked Questions
What is the current status of COVID vaccine availability in the US amid recent CDC changes?
The availability of COVID vaccines in the US has been affected by recent changes at the CDC, including a leadership shake-up. Despite these changes, the FDA has authorized updated COVID vaccines, and many pharmacies, including CVS, are currently offering vaccines, although some states have specific requirements such as needing a doctor’s prescription.
How do health policy changes impact COVID vaccine availability at pharmacies like CVS?
Health policy changes, particularly those stemming from CDC leadership updates, have led to delays in COVID vaccine availability at pharmacies like CVS. Some states are requiring additional approvals for pharmacists to administer vaccines, complicating access for individuals who need vaccinations.
What role does the FDA play in COVID vaccine availability during recent updates?
The FDA plays a crucial role in COVID vaccine availability, recently authorizing updated vaccines. However, new restrictions and requirements due to public health policies have created additional barriers for certain demographics, impacting how and when these vaccines can be administered.
How has the shake-up at the CDC affected public perceptions of COVID vaccinations?
The shake-up at the CDC, especially involving controversial figures like RFK Jr. and discussions around vaccine safety, has led to increased public scrutiny and uncertainty regarding COVID vaccinations. This could influence public willingness to receive vaccines amidst changing health policies.
What should the public know about upcoming COVID vaccination updates and their implications?
The public should stay informed about upcoming updates on COVID vaccinations from the CDC and FDA. It’s important to be aware of changing health policies and any potential delays or complications in availability, especially in light of the recent leadership changes at the CDC.
| Key Point | Details |
|---|---|
| New Acting Director | Jim O’Neill appointed as acting director of CDC after the dismissal of the previous director. |
| Background of O’Neill | O’Neill has previous experience at HHS from 2002 to 2008 and has degrees from Yale and the University of Chicago. |
| CDC Management Shake-Up | Resignations of top CDC scientists raise concerns about internal conflicts, particularly with David Geier’s controversial study on vaccines and autism. |
| Senate Concerns | Senator Bill Cassidy suggests postponing the ACIP meeting due to concerns about oversight and scientific rigor. |
| Vaccine Availability Issues | Limited FDA approval complicates COVID vaccine access at CVS, affecting several states. |
Summary
COVID vaccine availability is currently facing significant challenges due to recent developments at the CDC and FDA. The recent appointment of Jim O’Neill as the acting director of the CDC amid turmoil and the FDA’s limited approval for new vaccines highlight ongoing issues in vaccine distribution and access, particularly in key states like Massachusetts, Nevada, and New Mexico. These factors create obstacles for individuals seeking vaccination, further complicating public health efforts during the pandemic.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.