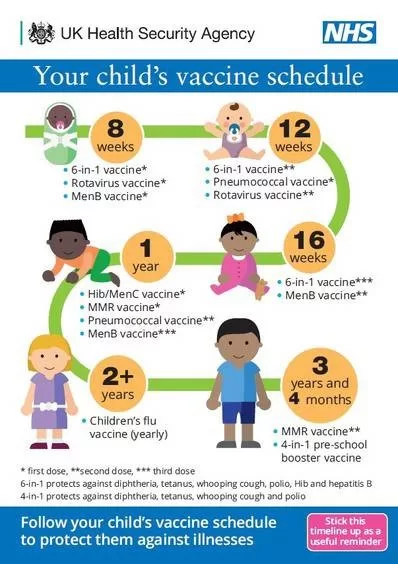
Children’s vaccine safety has taken a significant step forward with the recent reinstatement of the federal panel dedicated to overseeing the quality and efficacy of vaccines for young ones. The Task Force on Safer Childhood Vaccines, now active under the guidance of the Department of Health and Human Services (HHS), aims to ensure that childhood vaccinations are rigorous in both efficacy and safety. With increased focus on vaccine monitoring and research, this initiative seeks to address parental concerns regarding vaccine side effects and improve vaccine trust among families. Given the historical concerns raised by dissenting voices about the safety of routine childhood vaccines, the Task Force’s mission will be crucial in fostering a transparent dialogue. By committing to enhancing these vaccine safety systems, HHS hopes to reinforce public confidence in the vaccination process for children.
The issue of vaccine safety for children has become a focal point of national attention, particularly with the resurgence of initiatives aimed at ensuring the integrity of childhood immunizations. Recent developments, including the formation of the Safer Childhood Vaccines Task Force, emphasize an ongoing commitment to monitoring vaccine impacts and potential side effects. By prioritizing research into immune responses and reviewing vaccination schedules, stakeholders are working diligently to foster public assurance in the vaccination landscape. Addressing concerns that have arisen around childhood vaccines, this venture aims to enhance trust not only through oversight but also by promoting open discussions about vaccination risks and benefits. Such measures could play a pivotal role in alleviating doubts and fortifying the foundation of public health.
Understanding the Task Force on Safer Childhood Vaccines
The Task Force on Safer Childhood Vaccines has been reinstated by the Department of Health and Human Services (HHS) to enhance the monitoring of children’s vaccine safety. Originally established in 1986, this task force aims to provide comprehensive oversight regarding childhood vaccinations and their associated risks. Its reformation signifies a renewed commitment from federal authorities to ensure that vaccines administered to children not only meet public health standards but also prioritize the wellbeing of young patients.
This task force will collaborate with various health organizations, including the CDC and FDA, to deliver actionable recommendations for improving vaccine designs. By keeping the lines of communication open with the public and health professionals, it aims to bolster confidence in childhood vaccinations while systematically addressing any concerns related to vaccine side effects.
The Importance of Vaccine Monitoring for Children
Vaccine monitoring plays a critical role in ensuring the ongoing safety and efficacy of childhood vaccinations. By actively tracking adverse events following immunization, health authorities can quickly detect and respond to potential issues, thereby enhancing vaccine safety protocols. The Task Force will focus on refining these monitoring systems to ensure that they are efficient and transparent, ultimately reassuring parents about the vaccines their children receive.
Additionally, the task force’s initiatives will aim to improve the methods used for reporting any adverse reactions to vaccines. By increasing awareness and education surrounding vaccine side effects, the task force endeavors to foster a culture of safety that can further strengthen the community’s trust in vaccination programs.
Vaccine Safety Research: A Vital Component
As part of its mandate, the Task Force on Safer Childhood Vaccines is expected to actively engage in vaccine safety research. This research will not only focus on monitoring existing vaccines but will also seek potential improvements to minimize side effects associated with childhood vaccinations. Improved vaccine safety research is essential for identifying ways to decrease the risks while maximizing the benefits of immunizations.
Enhanced research partnerships will lead to data-driven decisions, benefiting both public health initiatives and pediatric care. In turn, this could result in more effective vaccines that inspire confidence among parents and caregivers about the safety and necessity of routine childhood vaccinations.
Addressing Concerns from Anti-Vaccine Advocates
The formation of this Task Force comes at a time when anti-vaccine advocates are increasingly vocal about their concerns over the safety of childhood vaccinations. They argue that the number of vaccines administered early in life could pose unforeseen risks to children’s health. The task force’s proactive approach attempts to address these concerns by implementing rigorous safety checks and open communication with the public.
By incorporating feedback from all stakeholders, including wary parents and health professionals, the Task Force aims to create a transparent vaccination process that builds trust and enhances community engagement. This supportive dialogue may help mitigate fears and misinformation surrounding vaccine safety.
Improving Trust in Vaccines through Transparency
Building public trust in vaccines is essential for successful immunization programs, and the Task Force is poised to play a significant role in this mission. By prioritizing transparency in their operations and findings, they can demonstrate accountability. Parents need to see a commitment to high standards of safety and a proactive approach to addressing potential side effects associated with vaccines.
Moreover, by releasing regular reports and updates about their findings, the Task Force can foster an environment where parents feel informed and engaged. Transparency not only enhances trust but also encourages the community to participate in ongoing discussions about vaccine safety, which is critical for the acceptance of childhood vaccinations.
The Role of Evidence-Based Recommendations
One of the core functions of the Task Force is to develop evidence-based recommendations concerning the safety and efficacy of childhood vaccines. These recommendations will rely on data collected from vaccine monitoring and safety research, ensuring that all decisions made are rooted in scientific integrity. The reliance on solid, empirical evidence is fundamental for the task force to enhance vaccine safety.
Furthermore, the task force’s efforts in advocating for sound scientific principles can create a ripple effect across the healthcare community, emphasizing the importance of evidence-based practices among healthcare providers. This could lead to improved dialogue about vaccine risks and benefits, equipping parents with the necessary information to make informed decisions.
New Framework for Evaluating Vaccine Safety
The Task Force on Safer Childhood Vaccines is expected to introduce a new framework that enhances the evaluation of vaccine safety. This framework will focus on the systematic assessment of adverse events linked to vaccinations. By continually updating and refining these processes, the task force aims to keep pace with advancements in medical science and changing public concerns about safety and efficacy.
Implementing such a robust framework will not only help in addressing the challenges brought up by skeptics but also fortify the arguments made by advocates of vaccines. A well-structured evaluation system can serve as a cornerstone for improving public health outcomes and ensuring that every child receives vaccines that account for their safety first.
Collaborative Efforts Between Agencies
The success of the Task Force will largely depend on collaboration between various health agencies, including the NIH, CDC, and FDA. Each organization brings distinct expertise and perspectives that can enrich the Task Force’s output. This level of collaboration is important for developing comprehensive strategies aimed at improving childhood vaccinations and ensuring that all recommended vaccines are safe for use.
Joint efforts can also streamline the vaccine approval process and ensure that safety protocols are consistently applied across the board. By coordinating among these agencies, the Task Force can effectively manage resources and focus on critical areas of research and monitoring that may require immediate attention.
Balancing Safety with Public Confidence
While improving vaccine safety is paramount, the Task Force must also balance this with maintaining public confidence in vaccines. There exists a potential risk that discussions surrounding enhanced vaccine monitoring may inadvertently fuel further skepticism among certain groups. To counter these concerns, the task force will need to communicate its findings and intentions clearly and engage with the community to address fears.
Building confidence in vaccines requires a delicate approach that not only involves addressing safety concerns but also highlighting the successes and benefits of vaccinations. The Task Force’s mission is to work towards minimizing fears while maximizing public health benefits, ensuring that childhood vaccinations are embraced as a vital component of healthcare.
Future Directions for Vaccination Policies and Practices
Looking ahead, the Task Force on Safer Childhood Vaccines will play a crucial role in shaping the future of vaccination policies and practices. Its approach to safety oversight will influence vaccine development processes and set new standards for ensuring that vaccines are safe for young populations. Policymakers will depend on the task force to deliver actionable insights that can guide future vaccination strategies.
As the landscape of vaccines evolves, the Task Force’s influence in ensuring that vaccination practices remain aligned with scientific evidence will be key. Continued efforts to improve vaccine safety not only safeguard children’s health but also help to cultivate a system where public trust in vaccines is nurtured and strengthened.
Frequently Asked Questions
What is the role of the Vaccine Safety Task Force in ensuring children’s vaccine safety?
The Vaccine Safety Task Force is designed to oversee and enhance the safety and quality of childhood vaccinations. It aims to develop, promote, and refine vaccines that minimize adverse reactions, collaborating with the CDC and FDA to support continuous vaccine safety research and improve adverse reaction reporting.
How does vaccine monitoring affect children’s vaccine safety?
Vaccine monitoring plays a critical role in ensuring children’s vaccine safety by tracking adverse reactions and identifying potential safety concerns in real-time. This ongoing surveillance helps health organizations maintain trust in vaccinations and ensures that any safety issues are promptly addressed.
What are common vaccine side effects children may experience?
Common vaccine side effects in children include mild reactions such as soreness at the injection site, low-grade fever, and irritability. Serious side effects are very rare. Understanding these potential side effects is essential for parents to make informed decisions about childhood vaccinations.
How is improving vaccine trust related to children’s vaccine safety?
Improving vaccine trust is closely tied to children’s vaccine safety, as transparency in reporting adverse reactions and ongoing safety research fosters public confidence in vaccination programs. When families feel assured that vaccines are being rigorously monitored for safety, they are more likely to participate in recommended vaccination schedules.
What steps is the HHS taking to enhance children’s vaccine safety?
The HHS is enhancing children’s vaccine safety by reinstating the Vaccine Safety Task Force, which will make recommendations for improving vaccine safety practices. This initiative aims to ensure that childhood vaccinations lead to fewer severe side effects while developing robust monitoring systems for ongoing safety research.
Why did concerns about children’s vaccine safety lead to the reinstatement of the Task Force?
Concerns raised by parents and advocates about the safety of routine childhood vaccinations and the cumulative effects of vaccines prompted the reinstatement of the Task Force. This group aims to rigorously examine and improve safety measures surrounding childhood vaccinations, addressing public fears and ensuring higher confidence in vaccine efficacy.
How will the Task Force on Safer Childhood Vaccines impact future vaccine recommendations?
The Task Force on Safer Childhood Vaccines will analyze current vaccination practices and recommend changes based on safety research findings. Its goal is to ensure that future vaccine recommendations are informed by rigorous safety data and public health needs, ultimately enhancing children’s vaccine safety.
| Key Point | Details |
|---|---|
| Reinstatement of Task Force | The HHS has reinstated the federal Task Force on Safer Childhood Vaccines to oversee children’s vaccine safety. |
| Mission | The Task Force aims to develop, promote, and refine vaccines to reduce adverse reactions. |
| Collaboration | It will work with other organizations like the CDC and FDA to support vaccine safety research. |
| First Report | The Task Force is expected to submit its first report to Congress within 2 years. |
| Concerns from Experts | Some experts worry that this move may undermine trust in existing childhood vaccines due to political motivations. |
| Anti-Vaccine Advocacy | Anti-vaccine groups claim routine vaccines’ safety has not been thoroughly studied, leading to public concerns. |
Summary
Children’s vaccine safety is a top priority for the Department of Health and Human Services. The reinstatement of the Task Force on Safer Childhood Vaccines highlights a renewed commitment to ensure children receive vaccines that are not only effective but also will lead to fewer adverse reactions. This Task Force will collaborate with other health organizations to bolster vaccine safety research and enhance the reporting of any vaccine-related adverse reactions. It is essential for parents to stay informed about these developments to build trust in the vaccination process.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.