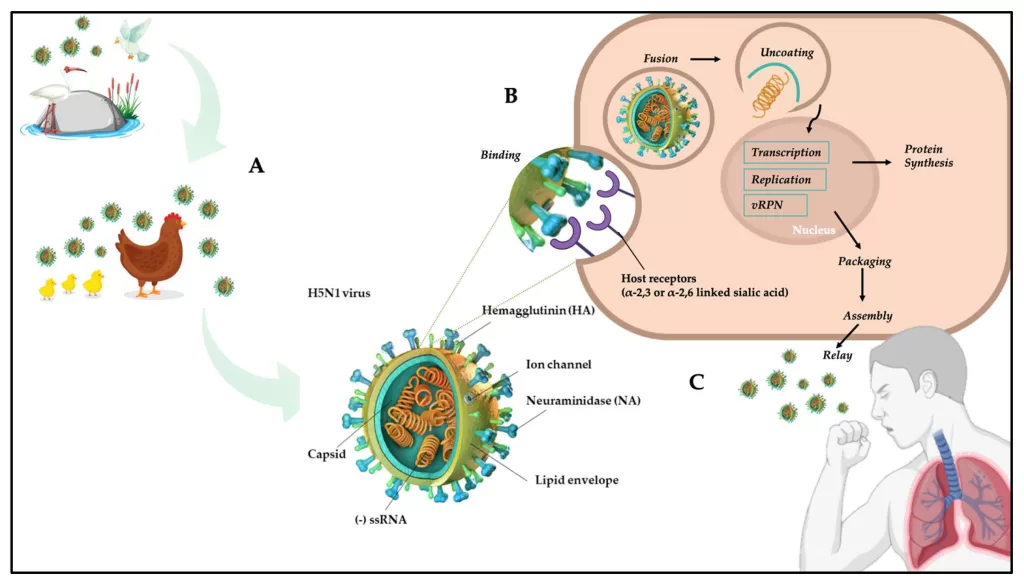
Avian Influenza H5N1 has emerged as a significant public health concern due to its highly pathogenic strains and potential for zoonotic transmission. This virus, particularly the H5N1 clade 2.3.4.4b, was recently identified in wild and domestic birds across Europe, marking alarming trends in avian influenza virus spread. The newly characterized EA-2023-DG genotype highlights the ongoing need for robust surveillance of avian influenza, especially as the virus undergoes frequent genetic reassortment. As we monitor these developments, understanding the transmission dynamics and potential spillover events is more critical than ever. Preventing the spread of this devastating disease requires a coordinated global approach in tracking and managing highly pathogenic avian influenza strains.
Bird flu, commonly referred to as avian influenza, poses a serious threat to both avian species and human health. Among its various strains, H5N1 has garnered particular attention due to its high pathogenicity and capacity to cross species barriers. Recent discoveries, including the novel EA-2023-DG genotype, underscore the evolving nature of these viruses, prompting a reevaluation of containment measures. The continuous emergence of new genetic variants emphasizes the importance of comprehensive surveillance programs aimed at curbing the spread of influenza among bird populations and mitigating potential outbreaks in humans. With increasing frequency of reassortment events, a multifaceted approach is paramount to monitor and address the impacts of avian influenza viruses.
Understanding the Emergence of H5N1 Clade 2.3.4.4b
The emergence of the H5N1 clade 2.3.4.4b virus represents a significant public health challenge in Europe. This highly pathogenic avian influenza (HPAI) strain has evolved through reassortment and genetic variation, notably when interacting with low pathogenicity avian influenza viruses. The EA-2023-DG genotype, identified in late 2023, showcases the complexities of virus evolution as it amalgamates genetic segments from various lineages, indicating a rich evolutionary history that poses risks to both avian and mammalian populations.
An understanding of the emergence of this novel reassortant highlights the importance of comprehensive surveillance strategies to monitor the H5N1 clade 2.3.4.4b. Continuous phylogeographic analyses help trace the virus’s routes of transmission and genetic diversification, particularly as migratory birds facilitate its spread across borders. Research indicates that the spread of H5N1 not only threatens domestic poultry but could also lead to a heightened risk of zoonotic transmission, warranting urgent attention from health organizations.
The Role of Wild Migratory Birds in Avian Influenza Spread
Wild migratory birds play a pivotal role in the epidemiology of avian influenza viruses, especially concerning the H5N1 clade 2.3.4.4b. These species act as natural reservoirs for the virus, facilitating long-distance translocation that can introduce these pathogens into new environments where susceptible domestic fowl reside. The tracking of migratory patterns is essential for predicting potential outbreaks, as these birds can harbor the virus without showing clinical signs, thereby enabling unnoticed transmission.
In light of the recent H5N1 outbreaks, enhanced surveillance among migratory bird populations has become critical. Identifying areas of high-risk migratory routes allows for targeted monitoring and rapid response measures. The linkage between wild bird movements and the spread of the EA-2023-DG genotype underscores the necessity of international cooperation in wildlife health initiatives, aiming to control the avian influenza virus spread before it reaches poultry farms and potentially impacts human health.
Genotyping and Surveillance of Avian Influenza Viruses
Genotyping methods are key in tracking the evolution and dispersion of avian influenza viruses, specifically for highly pathogenic strains like H5N1 clade 2.3.4.4b. The ongoing genomic surveillance allows for the identification of new genotypes such as EA-2023-DG, which aids researchers and public health officials in understanding its genetic makeup and potential impacts on both wildlife and agriculture. Continuous monitoring through sequencing databases like GISAID facilitates the collection and sharing of vital genomic data.
Furthermore, robust surveillance programs are integral to avian influenza management. They not only provide early warning systems for potential outbreaks but also help in assessing the effectiveness of biosecurity measures put in place within poultry industries. The dynamic nature of H5N1, evidenced by genetic reassortments, necessitates an adaptable approach to surveillance, focusing not only on wild populations but also on domestic poultry systems.
Phylodynamic Approaches to Monitor Avian Influenza
Phylodynamic methods provide invaluable insights into the evolutionary dynamics and spread of avian influenza viruses such as H5N1 clade 2.3.4.4b. By analyzing the genetic sequences and their geographic distribution, researchers can map out transmission pathways and potential hotspots for virus emergence. These insights enable public health authorities to devise strategic interventions tailored to the specific spread patterns and genetic characteristics of the virus.
In practice, applying phylodynamic analysis to the surveillance of HPAI facilitates informed decision-making in outbreak management. For instance, understanding the root causes of disease emergence and reassortment patterns can lead to timely vaccination campaigns and stricter biosecurity protocols at poultry farms. Emphasizing a proactive approach rather than a reactive one is essential to mitigating the risks posed by avian influenza outbreaks in both wildlife and domestic spheres.
Zoonotic Risks Associated with H5N1 Reassortants
The prospect of zoonotic transmission from the H5N1 clade 2.3.4.4b reassortant, particularly the EA-2023-DG genotype, heightens concerns for human health. Although the risk of direct spillover to humans remains relatively low, the genetic adaptability of the virus could facilitate such jumps, especially in immunocompromised individuals or those in close contact with infected animals. Surveillance systems must remain vigilant for human cases of avian influenza to promptly address any potential outbreaks.
Moreover, assessing zoonotic risks necessitates a multidisciplinary approach encompassing veterinary, ecological, and public health perspectives. This unified methodology can help identify health connections between avian influenza infections in birds and potential human health implications. Active measures including awareness campaigns on biosecurity and personal protective equipment usage can significantly reduce the exposure risk to individuals interacting with infected birds.
Impact of HPAI on Wildlife and Domestic Populations
The impact of highly pathogenic avian influenza (HPAI) on both wildlife and domestic bird populations is profound, particularly as the H5N1 clade 2.3.4.4b spreads across Europe. Birds affected by HPAI experience high mortality rates, leading to significant declines in wildlife populations and disruptions in ecosystems. Surveillance programs are crucial not only to understand the spread among domestic flocks but also to assess the health of wild avian communities and the potential consequences for biodiversity.
In the domestic sector, outbreaks of H5N1 can have catastrophic economic repercussions, resulting in culling of affected birds and strict containment measures. Farmers face not only the loss of livestock but also increased operational costs due to enhanced biosecurity measures. Any interconnections between wildlife health and poultry management policies need to be reevaluated in light of the recent outbreaks to protect both industries.
Lessons Learned from Previous Avian Influenza Outbreaks
Previous outbreaks of H5N1 and other avian influenza strains provide crucial lessons for managing current and future incidents. Understanding how past measures have failed or succeeded enables stakeholders to refine their strategies effectively. Instances of delayed responses, poor surveillance, and inadequate public awareness highlight the critical need for coordinated efforts across health sectors.
The historical data from avian influenza outbreaks emphasize the importance of preparedness and rapid response capabilities. Developing comprehensive plans that are regularly updated with new research findings from genetic monitoring can significantly enhance our response to emerging strains, such as the EA-2023-DG virus. Investing in research, public health infrastructure, and education will be fundamental to preventing future HPAI outbreaks.
The Future of Avian Influenza Surveillance
The future of avian influenza surveillance will likely include advanced genomic techniques and predictive modeling to anticipate the emergence of new strains like the H5N1 clade 2.3.4.4b. As we enhance our respiratory pathogen monitoring frameworks, integrating big data analytics and machine learning will become increasingly essential in identifying potential outbreaks before they escalate. This data-driven approach can leverage existing global networks and databases to provide robust, real-time information.
Furthermore, strengthening international collaboration will be crucial to combat the cross-border nature of avian influenza spread. A robust international alliance focusing on data sharing, joint surveillance programs, and coordinated response strategies can improve our overall preparedness. Investing in technology, infrastructure, and education across nations will bolster our collective ability to respond swiftly and effectively to future avian influenza challenges.
Frequently Asked Questions
What is Avian Influenza H5N1 and its implications for wildlife?
Avian Influenza H5N1, particularly the highly pathogenic avian influenza H5N1 clade 2.3.4.4b, poses significant threats to avian wildlife, causing severe morbidity and mortality. This strain has been detected frequently in wild birds and has undergone genetic reassortment, contributing to its rapid spread across Europe.
How is the H5N1 clade 2.3.4.4b virus spreading in Europe?
The H5N1 clade 2.3.4.4b virus, particularly the EA-2023-DG genotype, spreads through migratory wild birds and sporadic spillover events into domestic poultry. Surveillance of avian influenza indicates that this strain has rapidly disseminated from its initial detection in Germany across several European countries.
What is the significance of the EA-2023-DG genotype of Avian Influenza H5N1?
The EA-2023-DG genotype represents a notable reassortant variant of the H5N1 virus, combining segments from both highly pathogenic and low pathogenic avian influenza viruses. Its emergence emphasizes the need for continuous surveillance of avian influenza to manage potential outbreaks effectively.
What measures are in place for surveillance of avian influenza H5N1 in Europe?
Surveillance of avian influenza H5N1 in Europe involves ongoing monitoring of wild and domestic bird populations, genetic sequencing of H5 viruses, and phylogeographic analyses to track patterns of spread. These measures are crucial for early detection and intervention strategies against outbreaks.
What are the zoonotic risks associated with highly pathogenic avian influenza H5N1?
Highly pathogenic avian influenza H5N1 viruses, particularly new strains like EA-2023-DG, pose zoonotic risks as they can infect mammals, including humans. This raises concerns about public health and requires vigilant monitoring of cases among wildlife and poultry to prevent potential transmission to humans.
What have studies revealed about the origin and spread of the H5N1 EA-2023-DG virus?
Studies indicate that the H5N1 EA-2023-DG virus was first identified in a swan in Finland in November 2023, with its origins traced back to the southwestern Baltic Sea in late July 2023. Genetic analyses show this strain has rapidly spread throughout multiple European countries, necessitating robust surveillance and response strategies.
How can understanding the phylodynamics of avian influenza H5N1 aid in outbreak prevention?
Understanding the phylodynamics of avian influenza H5N1, such as the behaviors and genetic variations of strains like EA-2023-DG, enhances our ability to predict viral spread patterns, identify high-risk areas, and develop effective outbreak prevention and intervention strategies.
What role does genetic reassortment play in the evolution of H5N1 viruses?
Genetic reassortment plays a critical role in the evolution of H5N1 viruses by allowing them to acquire new genetic segments from other influenza viruses. This process, observed in strains like the EA-2023-DG genotype, increases viral diversity and can alter pathogenicity, making surveillance essential.
Why is it important to monitor the genetic diversity of avian influenza H5N1?
Monitoring the genetic diversity of avian influenza H5N1 is vital because increased genetic variation can lead to the emergence of new strains with different transmission and virulence characteristics. This knowledge is critical for developing effective vaccines and responding to potential outbreaks.
How does the movement of wild migratory birds contribute to the spread of avian influenza H5N1?
The movement of wild migratory birds is a primary factor in the spread of avian influenza H5N1, as these birds can carry and transmit the virus over long distances. Surveillance efforts focus on tracking these movements to prevent the virus from affecting domestic poultry and other species.
| Key Point | Description |
|---|---|
| Emergence of EA-2023-DG | The EA-2023-DG H5N1 clade was first detected in Germany in November 2023 after emerging from reassortment events involving low pathogenicity avian influenza viruses. |
| Geographic Spread | The virus quickly spread across 11 European countries, with notable cases reported in Germany, Sweden, and Poland. |
| Phylogeographic Analysis | Phylogenetic studies revealed the virus was circulating in northwestern Europe until June 2024, highlighting its extensive dispersal. |
| Zoonotic Risks | The dynamic reassortant nature of the EA-2023-DG strain raises concerns about its potential to infect humans, necessitating enhanced surveillance. |
Summary
Avian Influenza H5N1 has become a significant concern in Europe due to the emergence of the EA-2023-DG strain, identified as a clade 2.3.4.4b reassortant in late 2023. This virus demonstrates a capacity for rapid spread among both wild and domestic birds across multiple countries, underscoring its adaptability and potential risks to human health. Continuous phylogeographic analysis and enhanced surveillance are essential to understand its transmission dynamics and reduce the risks posed by this highly pathogenic pathogen.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.