Acute Hepatitis B infection, a serious viral illness, poses significant health risks, especially for patients undergoing treatments like acalabrutinib. This infection can lead to severe liver failure, necessitating urgent interventions, including transplantation. Despite vaccination against HBV, individuals can still experience reactivation of the virus, especially during immunosuppression. Studies indicate that patients receiving BTK inhibitors are at notable risk for such reactivation, underlining the need for careful monitoring. Vaccination against hepatitis B is critical, yet it may not guarantee full immunity, making awareness of potential liver complications increasingly vital.
Acute infection with the hepatitis B virus (HBV), often referred to as acute HBV hepatitis, is a pressing concern within the medical community. This viral disease can escalate into severe liver damage or even cirrhosis, particularly in immunocompromised patients undergoing therapies for conditions such as chronic lymphocytic leukemia. The risk of reactivation of HBV highlights the importance of vigilant serological monitoring, especially in patients previously vaccinated against the virus. As the prevalence of HBV remains high, tackling its impact is essential, particularly as it relates to treatment regimens involving immunosuppressive agents. Understanding the nuances of hepatitis B infection can lead to better patient outcomes and inform clinical practices.
Understanding Acute Hepatitis B Infection During Acalabrutinib Treatment
Acute hepatitis B virus (HBV) infection presents a significant health risk for patients undergoing treatments like acalabrutinib. This particular case highlights the precarious situation faced by even fully vaccinated individuals, as the rapid reactivation of HBV can lead to severe consequences such as liver failure. Despite prior vaccinations, the recognition of acute hepatitis B infection in previously seroprotected patients becomes essential in therapeutic settings. The mechanisms by which B-cell depleting therapies compromise protective immunity open a critical discussion regarding routine HBV monitoring among patients receiving such treatments.
In treating chronic lymphocytic leukemia with acalabrutinib, the risk of HBV reactivation increases substantially. Clinical manifestations of acute hepatitis B can include jaundice, abdominal pain, and a deterioration in the patient’s overall health, leading to dire circumstances like transplantation. Mechanistically, the immunosuppressive nature of acalabrutinib undermines the body’s ability to keep latent HBV under control, making awareness of this risk crucial for healthcare providers involved in cancer care.
The Role of Hepatitis B Vaccination in Cancer Patients
Vaccination against hepatitis B virus is a critical component of preventive healthcare, especially for patients with lymphoproliferative disorders. Ensuring adequate HBV antibody levels prior to starting immunosuppressive treatments can significantly mitigate the risks associated with HBV reactivation. Studies suggest that even patients who have been successfully vaccinated may experience a decline in immunity over time, particularly when undergoing intensive therapies like those involving Bruton tyrosine kinase inhibitors.
Current guidelines recommend comprehensive screening for HBV in patients before initiating therapies that may compromise immunity. This includes verifying the presence of protective anti-HBs antibodies and considering booster vaccinations when necessary to sustain immunity. As highlighted in this acute case of HBV infection, routine monitoring could potentially prevent severe clinical outcomes, including liver failure and the need for transplantation.
Potential Complications of HBV Reactivation
HBV reactivation in patients receiving acalabrutinib or similar therapies can lead to severe complications, including multi-organ failure and acute kidney injury. In the reported case, despite prior vaccination, the patient experienced rapid deterioration with hepatic and renal implications. Such complications underline the importance of early detection and timely intervention when managing patients whose immune systems may be compromised due to ongoing treatment.
In cases of HBV reactivation, the focus of treatment often shifts towards managing hepatic failure and its consequences. Interventions might include antiviral therapies, support for renal function, and preparation for possible liver transplantation. These measures require a multidisciplinary approach to care, highlighting the urgency of recognizing reactivated HBV as a critical consideration in the management of patients undergoing cancer treatment.
The Impact of Acalabrutinib on HBV Carriers
Acalabrutinib, a targeted therapy for chronic lymphocytic leukemia, carries a noteworthy implication for patients who are carriers of HBV. The potential for HBV reactivation necessitates that oncologists carefully screen for prior HBV exposure and immunization status before initiating treatment. Importantly, patients receiving acalabrutinib must be monitored for signs of liver strain and potential HBV actions, which may become more pronounced as treatment progresses.
Understanding the intersection between cancer therapies and HBV reactivation is imperative for patient safety. The clinical experience with patients who are both immunocompromised and virally active can inform future best practices, especially in how clinicians approach vaccination and HBV monitoring for at-risk populations undergoing treatment for hematologic malignancies.
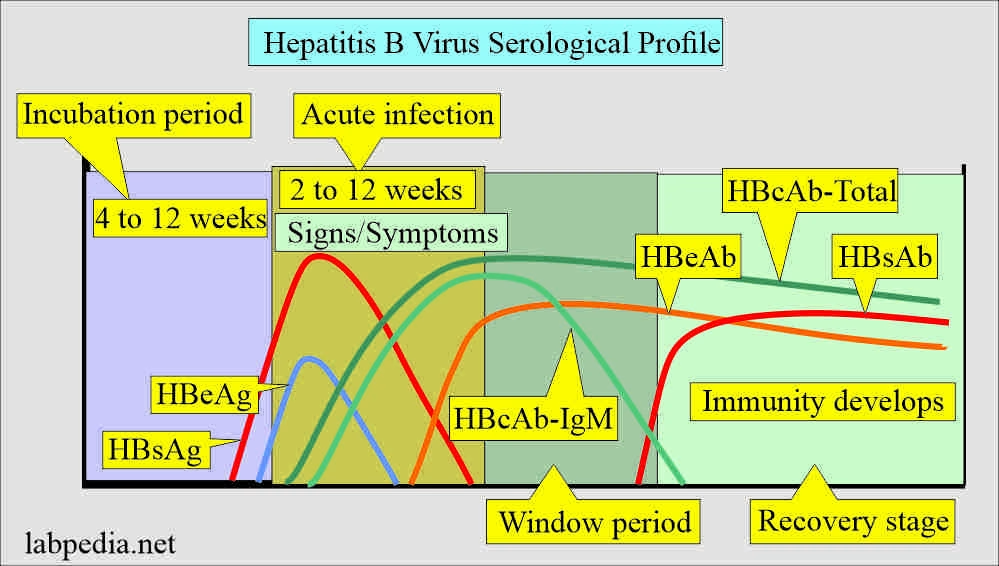
Monitoring HBV Antibodies in Immunosuppressed Patients
Monitoring HBs antibodies in patients undergoing immunosuppressive therapy is a point of contention. Most guidelines fail to recommend regular checks on HBs antibody levels, which may lead to oversights in patient care. The experience of the patient in the presented case serves as a cautionary tale, emphasizing that even with initial protective antibody levels, the immunity can weaken, placing patients at risk for acute hepatitis B infection.
Routine monitoring of HBV serology could provide critical insights into the patient’s immunity status, allowing healthcare providers to take proactive measures. Adjusting immunizations or extending monitoring protocols for patients undergoing therapies like acalabrutinib may help prevent severe outcomes associated with HBV reactivation and ensure greater patient safety throughout the treatment process.
Differential Diagnosis of Jaundice in Cancer Patients
Differential diagnosis of jaundice in patients undergoing cancer treatment must include acute hepatitis B infection, even in those with previous vaccination. It is a significant clinical indication that can alter treatment plans and expectations for recovery. As demonstrated in the case study, thorough evaluation for HBV should be standard practice in scenarios involving jaundice, particularly in patients receiving drugs like acalabrutinib that can impair immune function.
By integrating HBV considerations into the differential diagnosis for jaundice, clinicians can ensure timely interventions that may reduce serious complications. Moreover, educating healthcare teams about the links between antiviral therapy and HBV disease processes will lead to more comprehensive patient care and optimal treatment pathways.
Clinical Necessity of HBV Screening Before Immunosuppressive Therapy
The importance of thorough HBV screening before the initiation of immunosuppressive therapies cannot be overstated. Comprehensive serological testing plays a vital role in identifying patients at risk for HBV reactivation, ensuring that adequate precautions are taken. This includes assessing both HBsAg and HBs antibody levels to gauge a patient’s immunity. In cases where HBV exposure is confirmed, healthcare providers can formulate preventive strategies tailored to minimize the risk of reactivation.
The reported instance of acute hepatitis B infection amidst Acalabrutinib treatment indicates a gap in clinical protocol that must be addressed. Access to HBV screening and subsequent monitoring should be a fundamental aspect of care for patients at risk of reactivation due to immunosuppressive therapies, fostering a responsive and anticipatory healthcare environment.
Bruton Tyrosine Kinase Inhibitors and HBV Risks
As Bruton tyrosine kinase inhibitors like acalabrutinib serve as effective treatments for chronic lymphocytic leukemia, awareness of their association with HBV reactivation must be foregrounded. The unique mechanism of action inherent to BTK inhibitors often leaves patients vulnerable to opportunistic infections, including HBV. Therefore, thorough records of HBV vaccination history and immunity status are paramount before starting therapy.
In addition to careful screening, patient education surrounding the risks of HBV reactivation during BTK therapy can play a crucial role in preparedness. Health teams can proactively assess symptoms and manage potential complications associated with liver health, which is an essential step towards ensuring positive treatment outcomes.
Preventative Strategies Against HBV Reactivation
Effective strategies against HBV reactivation during the management of cancer patients are multi-faceted, involving both proactive screening and subsequent monitoring. Alongside vaccination efforts, utilizing antiviral prophylaxis in at-risk populations during immunosuppressive cytokine therapies can form crucial preventative layers. This approach could minimize the incidence of acute HBV infections and the associated morbidity seen in patients undergoing acalabrutinib treatment.
Another essential strategy involves continuous clinician education on the evolving landscape of HBV management in cancer treatment contexts. Incorporating regular updates to protocols regarding HBV screening, patient education, and symptomatic monitoring will bolster the clinical approach to prevent the reactivation of HBV and ensure that patients remain safe throughout their cancer treatment.
Frequently Asked Questions
What are the risks of HBV reactivation during acalabrutinib treatment for chronic lymphocytic leukemia?
Patients receiving acalabrutinib for chronic lymphocytic leukemia may experience HBV reactivation, even if they were previously vaccinated against hepatitis B. This increased risk is particularly concerning for individuals with a history of HBV exposure. Comprehensive HBV serologic screening is crucial before starting treatment to monitor for HBsAg and provide appropriate prevention strategies.
How does hepatitis B vaccination influence acute hepatitis B infection outcomes during immunosuppressive therapy?
Hepatitis B vaccination generally provides protective immunity; however, there are cases where vaccinated individuals may still develop acute hepatitis B infection during immunosuppressive therapies like acalabrutinib. It’s vital to regularly monitor HBs antibody levels in vaccinated patients to ensure maintained immunity and safeguard against potential HBV reactivation.
What are the symptoms of acute hepatitis B infection in patients treated with acalabrutinib?
Symptoms of acute hepatitis B infection in patients receiving acalabrutinib may include jaundice, abdominal pain, nausea, and anorexia. Laboratory tests often reveal elevated liver enzyme levels and presence of HBV markers, which necessitate immediate medical attention.
How is acute hepatitis B infection diagnosed in the context of immunosuppression?
In immunosuppressed patients, acute hepatitis B infection can be diagnosed through serology tests that detect HBsAg, HBc antibodies, and HBv DNA levels. Elevated liver enzymes and clinical symptoms are also evaluated to confirm the diagnosis of HBV infection.
What treatment options are available for acute severe hepatitis B infection in previously vaccinated patients?
Treatment for acute severe hepatitis B infection typically includes antiviral therapy such as entecavir, supportive care for liver function, and addressing complications like jaundice and liver failure. In severe cases, as seen with the patient treated with acalabrutinib, liver transplantation may be necessary.
Is there a link between chronic lymphocytic leukemia and HBV reactivation?
Yes, individuals with chronic lymphocytic leukemia have an intermediate risk of HBV reactivation, especially when undergoing treatments like acalabrutinib. This highlights the importance of evaluating HBV status and implementing proper monitoring and prophylactic measures.
What preventive measures are recommended for patients on BTK inhibitors regarding HBV?
Patients on BTK inhibitors, such as acalabrutinib, should undergo comprehensive HBV screening before initiating therapy. Ongoing monitoring of HBV antibody levels is also recommended to prevent loss of vaccine-induced immunity and manage any risk of HBV reactivation effectively.
Can previously vaccinated individuals develop acute hepatitis B infection?
Yes, previously vaccinated individuals can still develop acute hepatitis B infection, particularly under immunosuppressive conditions or therapies like acalabrutinib. Maintaining awareness of antibody levels and ensuring appropriate medical surveillance is crucial for preventing severe outcomes.
What is the role of HBs antibody monitoring during immunosuppressive therapy?
HBs antibody monitoring during immunosuppressive therapy is essential to assess the effectiveness of the hepatitis B vaccination and the risk of HBV reactivation. Regular checks can identify diminished immunity early, allowing for timely preventive interventions.
What are the implications of liver failure in patients with acute hepatitis B during treatment?
Liver failure in patients with acute hepatitis B can lead to severe complications, including the need for urgent liver transplantation as demonstrated in cases like those treated with acalabrutinib. Immediate medical intervention and supportive care are critical to managing the condition effectively.
| Key Points | Details |
|---|---|
| Acute Severe Hepatitis B Infection | A case of acute HBV infection with liver failure in a vaccinated patient treated with acalabrutinib. |
| Patient History | 58-year-old female; chronic lymphocytic leukemia; prior complete vaccination against HBV. |
| Symptoms at Admission | Severe jaundice, abdominal pain, nausea, signs of liver failure. |
| Diagnosis | Acute viral hepatitis B confirmed with PCR showing high HBV viremia. |
| Treatment | Entecavir, antibiotics, and support for liver failure. Liver transplantation was performed. |
| Guidelines and Recommendations | Importance of HBV screening before immunosuppressive therapy like acalabrutinib. |
Summary
Acute Hepatitis B infection can pose severe health risks, even in previously vaccinated individuals. This case report illustrates how a fully vaccinated patient developed acute severe hepatitis B virus infection while undergoing treatment with acalabrutinib. Despite being immunized, the patient experienced a significant deterioration in liver function, emphasizing the need for stringent monitoring and screening for HBV in patients receiving immunosuppressive therapies. Such occurrences underline the critical nature of ongoing vigilance in HBV management and the implications that immune reconstitution can have on previously acquired immunity.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.