Childhood vaccines are a crucial element in safeguarding the health of children, preventing numerous infectious diseases through effective immunization schedules. With the recent establishment of a new work group by the CDC’s Advisory Committee on Immunization Practices (ACIP), the focus on vaccine safety is at the forefront of public health discussions. This group aims to thoroughly review the efficacy and safety of childhood vaccinations, examining everything from the timing of doses to the potential risks associated with specific vaccine ingredients. As part of their mission, the work group will also seek to align CDC recommendations with rigorous scientific data and clinical insights. The findings are expected to shape future guidelines and reassure parents about the importance of adhering to the childhood immunization schedule, which plays a vital role in protecting both individual children and the broader community.
In the field of pediatric health, immunizations are essential tools to ensure the wellbeing of younger populations, protecting them from a range of preventable diseases. The CDC is currently taking steps to address concerns surrounding these vaccinations, particularly through the assembly of a dedicated panel that will scrutinize various aspects of the vaccination approach for children. This endeavor aligns with health authorities’ commitments to review and enhance the immunization protocol based on comprehensive safety assessments and cutting-edge research. Furthermore, discussions by health experts such as those within the ACIP are centered on improving vaccine safety while maintaining robust communication with parents regarding the benefits of early immunization. Such initiatives are vital in fostering trust and encouraging the continued success of childhood vaccination programs.
Understanding Childhood Vaccines and Their Importance
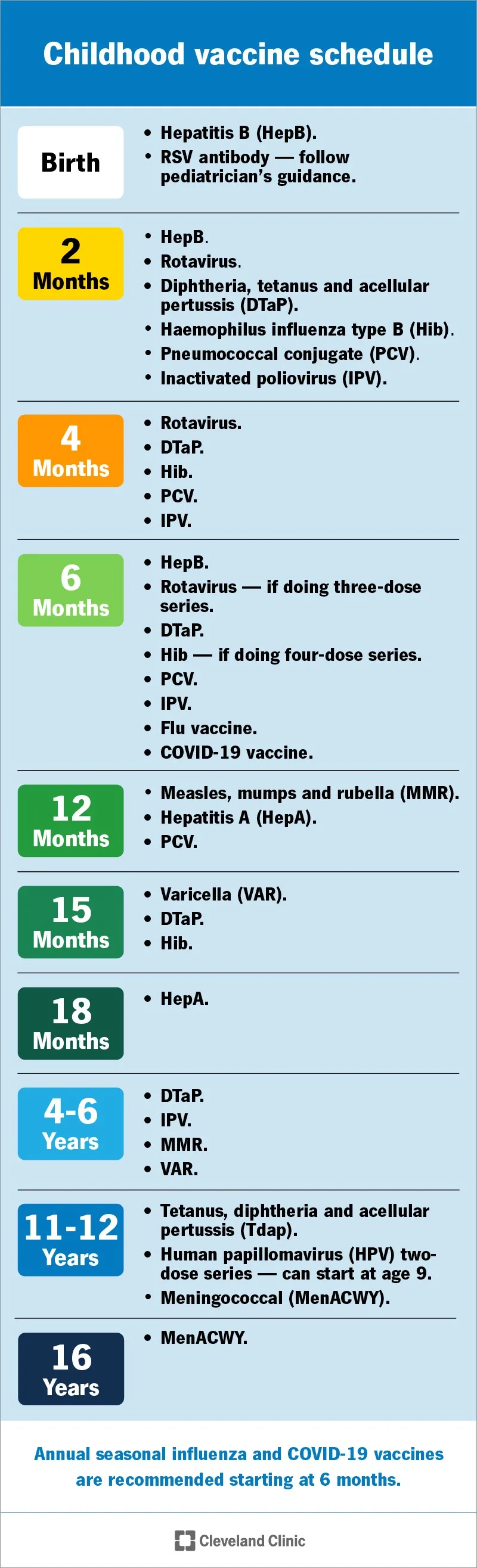
Childhood vaccines play a crucial role in public health by preventing serious diseases and protecting communities. The childhood immunization schedule, as recommended by the Centers for Disease Control and Prevention (CDC), serves to ensure that children receive vaccines at the right times to build immunity effectively. Immunizations save millions of lives each year and contribute to herd immunity, which protects those who cannot be vaccinated for medical reasons. By adhering to the CDC recommendations, parents can safeguard their children and assist in controlling the spread of infectious diseases.
In recent discussions, the Advisory Committee on Immunization Practices (ACIP) has highlighted the ongoing necessity for a thorough review of childhood vaccines. This review not only examines the safety and efficacy of the vaccines but also evaluates how different schedules and combinations of vaccines impact overall health. Understanding the science behind these vaccines, including their ingredients and the timeline in which they are administered, is essential for families to make informed decisions about immunization.
The Role of the CDC and ACIP in Vaccine Recommendations
The CDC and the Advisory Committee on Immunization Practices (ACIP) play pivotal roles in formulating vaccine recommendations in the United States. ACIP evaluates data on vaccine safety and efficacy and provides guidelines that the CDC enforces nationwide. The recent establishment of the Childhood and Adolescent Immunization Schedule Workgroup reflects ACIP’s commitment to reassess the existing immunization schedule based on current scientific literature and clinical findings. As vaccine debates continue, the credibility and transparency of these recommendations remain essential to maintain public trust.
Additionally, the involvement of external experts in these workgroups ensures that a diverse array of opinions is considered when reviewing vaccine safety. The workgroup will analyze various topics, including the timing and combined administration of vaccines, which reflects ACIP’s proactive approach to adapt to new evidence concerning vaccine methodologies. Such initiatives are crucial for addressing public concerns about childhood vaccinations while reinforcing community health.
Debates Surrounding Vaccine Safety and Efficacy
Debates about vaccine safety often arise in the public domain, particularly concerning childhood vaccines. Critics argue that the current immunization schedule could be linked to various health challenges faced by children today, although these claims are generally not substantiated by robust scientific evidence. The establishment of the new workgroup by ACIP showcases a willingness to discuss these safety concerns openly and responsibly, ensuring that all data pertaining to vaccine safety—including potential risks of ingredients like aluminum—are scrutinized.
Furthermore, the historical context of vaccines in American healthcare emphasizes the importance of rigorous evaluation. Vaccine safety has been established through decades of research, continuous monitoring, and surveillance systems that track adverse events. Despite the persistent skepticism portrayed in media and by select public figures, the consensus among health authorities remains that the benefits of vaccinations far outweigh their risks.
Challenges to the Childhood Immunization Schedule
As ACIP continues to evaluate the childhood immunization schedule, challenges arise relating to public perception and misinformation surrounding vaccines. High-profile figures advocating for changes in the immunization schedule, including suggestions to separate the combined measles, mumps, and rubella (MMR) vaccine, have contributed to confusion among parents. Such shifts could lead to hesitancy toward vaccinations, as parents may hold back on immunizing their children due to concerns about the safety of current recommendations.
Moreover, the potential disruption of long-established immunization practices poses a significant challenge for public health initiatives. Experts assert that the medical evidence strongly supports the combination of vaccines as being safe and effective. Hence, any proposed changes to the schedule need to be grounded in credible research and not merely anecdotal claims. Continuous education and transparent communication from healthcare providers are critical to combatting misinformation and fostering confidence in the vaccination process.
The Importance of Community Immunity
Community immunity, or herd immunity, is vital for protecting those who cannot be vaccinated, such as infants and individuals with certain medical conditions. When the majority of the population is vaccinated against specific diseases, it diminishes the likelihood of disease outbreaks. Childhood vaccinations contribute significantly to this collective protection, ensuring that vulnerable groups are shielded from contagious illnesses. Public health initiatives emphasize not just individual vaccine decisions, but also the broader implications for community health.
A well-implemented immunization schedule, as endorsed by the CDC and ACIP, facilitates widespread community immunity. However, with increasing public debates about vaccine safety, it is essential to communicate the necessity of maintaining high vaccination rates. This connection between individual choices and community health outcomes reinforces the importance of adhering to the recommended vaccination schedule for childhood immunizations.
Continuous Review and Improvement of Vaccination Policies
The ongoing review process of vaccination policies is crucial for adapting to new scientific evidence and public health needs. The establishment of the Childhood and Adolescent Immunization Schedule Workgroup signifies a commitment from the ACIP to ensure that immunization practices evolve based on the latest research in vaccine safety and efficacy. As new vaccines are developed, and more data becomes available about existing ones, these recommendations will likely continue to change.
This proactive approach to reviewing vaccination guidelines not only reflects the CDC’s dedication to public health but also reinforces trust in the system. Families are encouraged to stay informed about these changes and understand that their children’s health is prioritized in these discussions. Open dialogue about vaccine policies and ongoing research can help prevent misinformation from undermining public confidence.
Responding to Vaccine Hesitancy
Vaccine hesitancy, fueled by misinformation and societal debates, poses significant challenges to immunization efforts. Addressing these concerns requires a multi-faceted approach that involves healthcare providers, public health advocates, and the dissemination of accurate information regarding vaccine safety. It is essential to foster conversations that allow families to express their concerns while providing evidence-based responses that highlight the importance of childhood vaccinations.
Engaging with communities and understanding their hesitancy can help tailor outreach efforts effectively. Public health campaigns need to focus on building trust and informing parents about the benefits of the childhood immunization schedule as recommended by the CDC and ACIP. Strategies to reduce vaccine hesitancy can include accessible education, transparent communication, and visible endorsements from trusted healthcare professionals.
Global Perspectives on Childhood Vaccinations
Examining global perspectives on childhood vaccinations can provide valuable insights into best practices and effective strategies for immunization programs. Different countries implement varied schedules and vaccine combinations, which can influence the safety and efficacy of their approaches. By analyzing how other nations address challenges related to vaccine acceptance and public health outcomes, the ACIP may gain useful perspectives when considering any prospective modifications to the immunization schedule.
Furthermore, collaboration with international health organizations can inform CDC recommendations and help align U.S. vaccine strategies with global standards. A comprehensive understanding of childhood vaccines from a global viewpoint can enhance local immunization efforts and ensure that children receive the best protection possible against preventable diseases.
The Future of Vaccine Development and Policy
As science and technology advance, the future of vaccine development holds great promise for improving public health outcomes. Innovations in vaccine formulation, delivery methods, and safety monitoring can help address the concerns of vaccine hesitancy while enhancing the efficacy of childhood vaccinations. Organizations like ACIP will play critical roles in evaluating and recommending new vaccines that are introduced to the childhood immunization schedule.
Future vaccine policies will need to adapt as new challenges arise, such as emerging infectious diseases and shifts in public opinion about vaccinations. Continued research, technological advancements, and community engagement will be essential in shaping a robust vaccination framework that not only protects individual health but also fortifies public health systems in the U.S.
Frequently Asked Questions
What is the role of the CDC in childhood vaccines and immunization schedules?
The Centers for Disease Control and Prevention (CDC) plays a crucial role in establishing the childhood immunization schedule, which outlines the recommended childhood vaccines, their timing, and dosages. This schedule is developed based on research and recommendations from the Advisory Committee on Immunization Practices (ACIP), which evaluates vaccine safety and efficacy to inform public health policies.
How safe are childhood vaccines according to current research?
Current research, including studies reviewed by the CDC and ACIP, indicates that childhood vaccines are generally safe and effective. Extensive safety monitoring systems in place, like the Vaccine Adverse Event Reporting System (VAERS), help ensure any potential issues are swiftly identified and addressed, supporting the continued recommendation of the childhood vaccination schedule.
What updates have been made to the childhood immunization schedule recently?
Recent updates to the childhood immunization schedule have included reevaluations of specific vaccines, such as the recommendation to administer the combined measles, mumps, rubella, and varicella (MMRV) vaccine separately. This is part of ongoing assessments from the CDC and ACIP workgroups aimed at optimizing vaccine safety and effectiveness.
What factors does the ACIP consider when reviewing childhood vaccines?
The ACIP considers various factors when reviewing childhood vaccines, including the timing and order of vaccinations, the safety of vaccine components, effectiveness of the vaccines, and the surveillance of post-vaccination health outcomes. This comprehensive analysis informs their recommendations on the childhood immunization schedule.
Are there concerns about the timing of childhood vaccinations?
Yes, there are ongoing discussions regarding the timing of childhood vaccinations. The ACIP workgroup is currently analyzing data about the combined administration of vaccines and whether certain vaccines should be sequenced differently to minimize the risk of adverse effects, such as febrile seizures.
How does the CDC communicate changes to the childhood vaccination recommendations?
The CDC communicates changes to childhood vaccination recommendations through public announcements, updates to the immunization schedules on their website, and through information distributed to healthcare providers and public health officials, ensuring that parents and guardians are well-informed about childhood vaccines.
What ongoing research is being conducted on childhood vaccines?
Ongoing research on childhood vaccines includes examining the long-term effects of vaccines, the safety of various ingredients (like aluminum in some vaccines), and assessing the effectiveness of current vaccination strategies. This research is critical for maintaining the integrity and safety of the childhood immunization schedule managed by the ACIP and CDC.
What is the significance of workgroups like the Childhood and Adolescent Immunization Schedule Workgroup (WG)?
Workgroups like the Childhood and Adolescent Immunization Schedule Workgroup (WG) are significant in conducting detailed reviews of childhood vaccines. They analyze clinical and scientific data to provide evidence-based recommendations to the ACIP, helping to ensure that the immunization schedule reflects current research and promotes public health.
How do childhood vaccines impact public health according to CDC recommendations?
According to CDC recommendations, childhood vaccines significantly impact public health by preventing the spread of infectious diseases, reducing illness and hospitalization rates, and ultimately contributing to community immunity. This collective protection is essential in keeping vulnerable populations safe.
What challenges are currently facing the childhood vaccination schedule?
Current challenges facing the childhood vaccination schedule include addressing vaccine hesitancy, ensuring equitable access to vaccines, and ongoing scrutiny from vaccine critics. The CDC and ACIP are actively working to communicate the benefits of childhood vaccinations and to incorporate new scientific insights into their policies.
| Key Points |
|---|
| A new work group has been created by the CDC to review the childhood immunization schedule, focusing on safety and efficacy. |
| The work group will assess vaccine timing, safety of ingredients, and other countries’ schedules. |
| ACIP has included members who are vocal critics of the current vaccine schedule. |
| Recent changes discussed include separating the MMR vaccine and reviewing the DTaP vaccine administration timing. |
| HHS has reactivated a prior task force to evaluate vaccine safety, but restricted certain expert participation. |
| Continued scrutiny is being placed on aluminum and other vaccine components. |
| There are concerns that public figures may influence parents’ perception of vaccine safety. |
Summary
Childhood vaccines are critical for ensuring the health and well-being of children by preventing serious diseases. Recently, the CDC’s Advisory Committee on Immunization Practices established a work group to scrutinize the immunization schedule’s safety and effectiveness, a move that highlights ongoing debates and developments in vaccine administration. This new initiative underscores the importance of maintaining an evidence-based approach to vaccines, which are crucial for public health and preventing outbreaks of dangerous diseases.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.