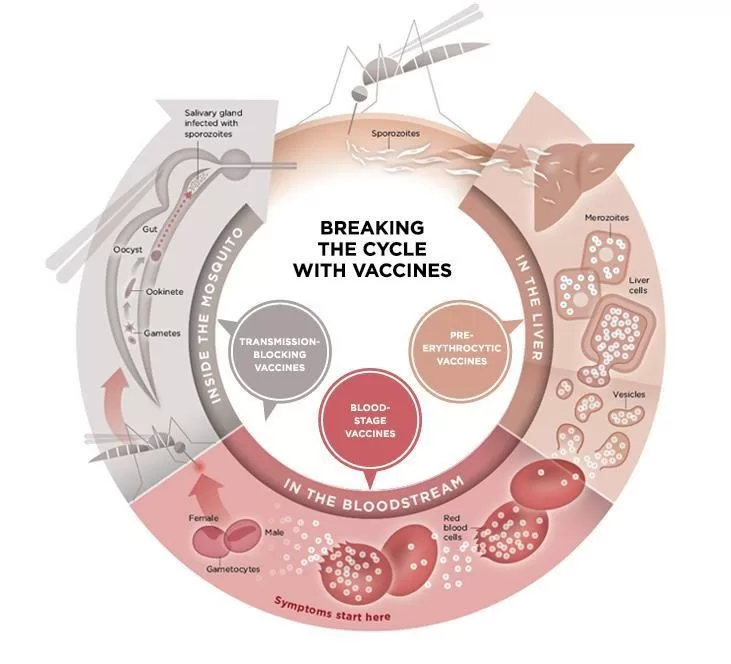
The introduction of the preconception malaria vaccine represents a promising advancement in malaria prevention efforts, especially in regions where the disease poses significant risks to maternal health. This innovative strategy is aimed at immunizing women before pregnancy, reducing the incidence of malaria infections known to contribute to 50,000 maternal deaths and 200,000 stillbirths annually in Africa. Recent trials utilizing the PfSPZ vaccine have shown effective results in protecting women against malaria, which could revolutionize prenatal care and vaccination before pregnancy. With a focus on this groundbreaking malaria vaccine trial, researchers are striving to ensure safer pregnancies and healthier newborns. The findings from such studies underscore the urgent need for effective malaria vaccines as a cornerstone for improving maternal health outcomes in vulnerable populations.
Exploring new avenues in malaria control, the concept of a malaria vaccine administered prior to conception could drastically alter preventive strategies in endemic areas. This initiative focuses on enhancing maternal immunity and safeguarding both mothers and newborns from the detrimental effects of malaria during pregnancy. By leveraging recent findings from clinical trials involving the PfSPZ vaccine, researchers are forging paths toward innovative vaccination practices, where women are immunized against malaria well before they conceive. This proactive approach is crucial in tackling the severe implications malaria has on maternal health, which often goes unaddressed with conventional prevention methods. As we move forward, the development of such vaccines not only addresses the immediate health of women but also paves the way for healthier generations to come.
The Role of Malaria Prevention in Maternal Health
Malaria prevention is critical for safeguarding maternal health, especially in regions where the disease is endemic. In many African countries, malaria infections during pregnancy can lead to dire consequences for both the mother and the fetus. As reported, malaria accounts for thousands of maternal deaths and stillbirths each year, making effective prevention strategies a necessity. Reliable tools, such as bed nets and preventive treatment, have fallen short, prompting researchers to explore alternatives that can significantly reduce these health risks.
One promising approach that has emerged is vaccination prior to conception. This strategy not only incentivizes prospective mothers to consider their health before pregnancy, but it also represents a significant advancement in malaria prevention technology. The integration of innovative vaccines into maternal health strategies could potentially change the landscape of maternal and neonatal care in malaria-prone areas.
Insights from the Preconception Malaria Vaccine Trial
The recent findings from the preconception malaria vaccine trial offer a hopeful outlook for malaria prevention strategies. Conducted by researchers at the National Institutes of Health in collaboration with institutions in Mali, the trial investigated the safety and efficacy of the PfSPZ vaccine. The results indicate that immunizing women before they conceive can significantly decrease the incidence of malaria infections during pregnancy, thereby lowering the risks associated with maternal and fetal health.
This trial is noteworthy not just for its findings on vaccine efficacy—65% for the low dose and an impressive 86% for the high dose—but also for its comprehensive approach to monitoring maternal health. The prolonged monitoring period, extending over two years, provided vital insights into the vaccine’s durability of protection without the need for booster doses. This evidence lays the groundwork for future research that aims to assess the vaccine’s effects during pregnancy, which holds promise for improving outcomes for mothers and their newborns.
Implications for Malaria Vaccine Trials and Maternal Health
As the recent trial outcomes demonstrate, incorporating women of childbearing age in malaria vaccine trials is essential for enhancing maternal health strategies. Traditionally, pregnant women have been excluded from clinical trials due to safety concerns, limiting our understanding of how vaccines may protect both mothers and infants. The insights gained from this trial, including the demonstrated safety of the PfSPZ vaccine, challenge the status quo and underscore the necessity for policy changes that support the inclusion of pregnant women in future studies.
The data from this trial not only highlight the importance of preconception vaccinations but also serve as a catalyst for advancing maternal health initiatives. With solid evidence around the vaccine’s safety and efficacy, we can advocate for more integrated approaches to malaria prevention that emphasize pre-pregnancy immunization and protective healthcare measures. This could pave the way for more comprehensive maternal health policies that prioritize the well-being of both mothers and their children.
The Future of Malaria Vaccines and Maternal Health
Looking ahead, the future of malaria vaccines, particularly in the context of maternal health, appears increasingly promising. The favorable results from the preconception malaria vaccine trial using the PfSPZ vaccine highlight the potential for these immunizations to be integrated into standard healthcare protocols for women planning to conceive. Such integration could profoundly impact malaria prevention, ultimately leading to fewer maternal deaths and stillbirths in at-risk populations.
Further studies are necessary to fully understand the implications of the vaccine during pregnancy and to refine protocols for its administration. A larger-scale study will provide critical data that can inform public health strategies and vaccination schedules tailored to women in malaria-prone regions. This foundational research is pivotal for developing comprehensive malaria vaccination programs that address maternal health holistically.
Understanding the Importance of Vaccination Before Pregnancy
The notion of vaccination before pregnancy is gaining traction, particularly as research demonstrates its potential benefits in reducing malaria infections among expectant mothers. By vaccinating women prior to conception, healthcare providers can create a protective barrier that not only improves maternal health but also enhances fetal outcomes. This proactive approach represents a significant shift toward preventive healthcare, addressing the need for effective malaria interventions before the onset of pregnancy.
Moreover, integrating vaccination into preconception care aligns with global health goals to reduce maternal and child mortality rates. Investing in preconception health is crucial, as many women may be unaware of their malaria risk or the implications of infection during pregnancy. By raising awareness and providing accessible vaccination options, we can empower women to take charge of their health and reduce the prevalence of malaria-related complications in their pregnancies.
Concluding Thoughts on Malaria Vaccine Development
In conclusion, the ongoing research into malaria vaccines, specifically the preconception malaria vaccine trial, opens new avenues for addressing maternal health challenges. With significant advancements in vaccine technology, like the PfSPZ vaccine, we may be on the brink of a revolutionary change in how we approach malaria prevention among women of childbearing age. This progress not only highlights the importance of continued investment in malaria vaccine trials but also reinforces the need for tailored health strategies that consider the unique needs of mothers.
The path ahead will involve further trials to ensure that these vaccines are safe and effective in various populations, particularly pregnant women. Ultimately, successful implementation of the lessons learned from the trial could lead to enhanced maternal health outcomes and provide a powerful tool in the global struggle against malaria.
Frequently Asked Questions
What is the preconception malaria vaccine and how does it relate to malaria prevention?
The preconception malaria vaccine refers to the PfSPZ vaccine, an investigational vaccine designed to immunize women before pregnancy to prevent malaria infections that can adversely affect maternal health and fetal outcomes. This approach aims to reduce cases of malaria during pregnancy, which is linked to numerous maternal deaths and stillbirths, particularly in regions with high malaria prevalence.
How effective is the PfSPZ vaccine in reducing malaria infections in women trying to conceive?
In recent trials, the PfSPZ vaccine demonstrated notable effectiveness in malaria prevention. Among women who received the vaccine, efficacy rates were recorded at 65% for the lower dose and 86% for the higher dose in preventing malaria during preconception. This highlights the vaccine’s potential to protect women from malaria infections as they prepare for pregnancy.
What safety measures have been taken in the trials for the preconception malaria vaccine?
The trials for the preconception malaria vaccine, particularly the PfSPZ vaccine, have prioritized safety for both women and their newborns. Initial findings indicate that the vaccine is safe to administer prior to pregnancy and does not compromise maternal or fetal health, establishing confidence for its broader use in malaria prevention strategies.
What are the implications of the findings from the preconception malaria vaccine trial on maternal health?
The findings from the preconception malaria vaccine trial are promising for maternal health. With the vaccine showing durable protection without the need for frequent boosting, it could significantly lower the risk of malaria infections in pregnant women, potentially alleviating maternal and neonatal health challenges posed by malaria.
What future research is planned for the PfSPZ malaria vaccine in relation to pregnancy?
Future research will focus on testing the PfSPZ malaria vaccine’s safety during pregnancy and its overall effectiveness when administered prior to conception. Larger studies are anticipated to build on current data, emphasizing the importance of protecting maternal health and improving outcomes for mothers and their babies.
How does vaccination before pregnancy potentially change malaria prevention strategies?
Vaccination before pregnancy, specifically with the preconception malaria vaccine, introduces a proactive approach to malaria prevention. By immunizing women ahead of conception, this strategy aims to reduce malaria incidence rates in pregnancy, thereby improving maternal and fetal health outcomes, and offering new avenues for public health interventions in malaria-endemic areas.
What were the specific results of the malaria vaccine trial in Mali?
In the malaria vaccine trial conducted in Mali, the PfSPZ vaccine was administered to 300 women, with substantial results. The average efficacy was found to be 65% for the lower dose and 86% for the higher dose among women who became pregnant shortly after vaccination, demonstrating substantial potential for reducing malaria infections in women of childbearing age.
| Key Points | Details |
|---|---|
| Importance of Preconception Malaria Vaccine | Malaria during pregnancy leads to significant maternal and fetal health issues, accounting for 50,000 maternal deaths and 200,000 stillbirths annually in Africa. |
| Trial Overview | Conducted by the NIH and partners in Mali with 300 women planning to conceive, testing an investigational vaccine called PfSPZ. |
| Vaccine Safety | Vaccination proved safe for women and infants, with no adverse effects reported. |
| Efficacy Rates | Efficacy was 65% for the low dose and 86% for the high dose during the first year. In total, 57% and 49% efficacy observed for pregnancies during both years. |
| Durable Protection | The vaccine provided durable protection for two years without the need for boosters, distinguishing it from other malaria vaccination methods. |
| Future Research | Next phase will investigate safety during pregnancy and expand studies on its administration before conception. |
| Research Significance | The trial framework is significant for future evaluations in pregnant women, traditionally excluded from such studies. |
Summary
The preconception malaria vaccine presents a revolutionary approach to preventing malaria infections in pregnant women, aiming to enhance maternal and fetal health outcomes significantly. By immunizing women before pregnancy, this innovative strategy not only addresses the high rates of maternal deaths and stillbirths caused by malaria in Africa but also demonstrates promising efficacy and safety. As researchers move forward with larger studies, the potential for this vaccine to change tropical health dynamics is becoming increasingly recognized.
The content provided on this blog (e.g., symptom descriptions, health tips, or general advice) is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website. If you believe you may have a medical emergency, call your doctor or emergency services immediately. Reliance on any information provided by this blog is solely at your own risk.